Recently, Cell Death&Disease published an article written by Wang Weibin, a professor of Peking Union Medical College Hospital, who said that the team found that the Inner mitochondrial membrane protein STOML2 can inhibit the proliferation and drug resistance of pancreatic cancer cells through the ingeniously designed multi-dimensional and multi type tests, suggesting that STOML2 gene is expected to become a new therapeutic target.
Pancreatic cancer is one of the most unfavorable cancers with a 5-year survival rate of less than 12%. Surgical resection of the lesion and postoperative chemotherapy supplemented with Gemcitabine are the main methods for clinical treatment of pancreatic cancer. However, Gemcitabine is prone to drug resistance, which affects the survival time of patients. The researchers said that STOML2 gene at 9p13.1 is involved in encoding STOML2 protein, which is highly expressed in pancreatic cancer cells, and may be related to the drug resistance of pancreatic cancer.
Previous studies have shown that STOML2 protein can promote the progression of many cancers, including pancreatic cancer. However, pancreatic cancer is fundamentally different from other malignant tumors in biology, and the previous research data on STOML2 protein in pancreatic cancer is limited and the evidence is insufficient, so the academic community is still unclear about the specific function and mechanism of STOML2 protein in drug resistance of pancreatic cancer.
To this end, the research team has designed multiple dimensions and types of experiments to systematically and perfectly define the biological role and significance of STOML2 in pancreatic cancer cells. Pathological tissue microarray detection found that the high expression of STOML2 protein in pancreatic cancer patients was positively related to the higher five-year survival rate. Cell proliferation test and cytotoxicity test of chemotherapy drugs found from the cytological level that STOML2 protein can inhibit the proliferation of pancreatic cancer cells and increase the chemotherapy effect of Gemcitabine.
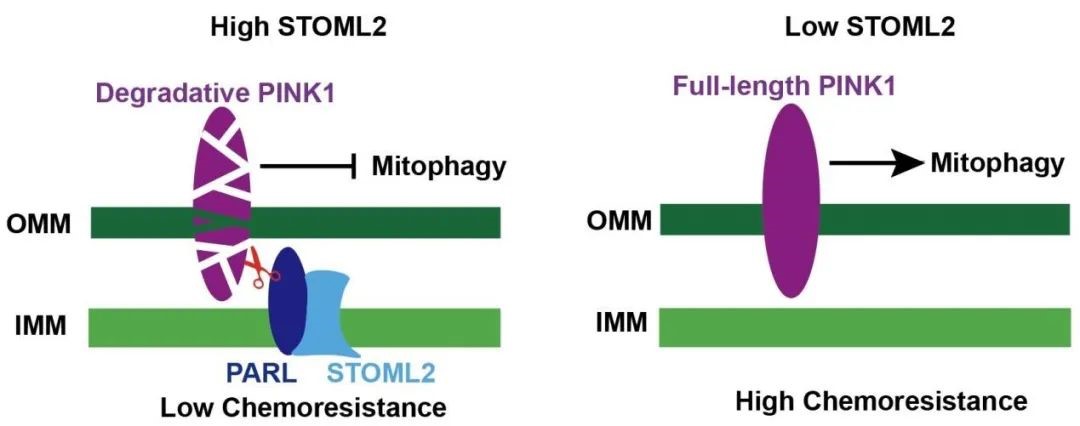
The co immunoprecipitation experiment and the upstream and downstream rescue experiment are clear from the molecular biology level. Through the PARL/PINK1 pathway, STOML2 protein can specifically stabilize the PARL protein on the Inner mitochondrial membrane and reduce mitochondrial autophagy (the targeted degradation of mitochondria by cells). A large number of mitochondria damaged by Gemcitabine accumulate in the cells, producing excessive reactive oxygen species (ROS), and finally cooperate with chemotherapy drugs to induce apoptosis of pancreatic cancer cells and improve the chemotherapy effect.
The nude mice subcutaneous tumor transplantation experiment simulated the human pancreatic cancer environment, and verified the researchers' guess: compared with the control group, the tumor volume of the STOML2 protein overexpression group mice was significantly reduced, directly proving that STOML2 protein can enhance the chemotherapy effect of Gemcitabine.
Wang Weibin said that these studies on the acquisition and loss of functions in vitro and in vivo, as well as in vivo combined therapy, showed that STOML2 protein plays an important role in mitochondrial autophagy, can accelerate the apoptosis process of pancreatic cancer cells, and improve the chemotherapy effect. This study provides an important theoretical basis for the development of STOML2 protein as a potential Targeted therapy site.
STOML2 restricts mitophagy and increases chemosensitivity in pancreatic cancer through stabilizing PARL-induced PINK1 degradation
Journal:Cell Death & Disease
Corresponding Author:Weibin Wang
Abstract:
Pancreatic cancer remains one of the most lethal diseases with a relatively low 5-year survival rate, and gemcitabine-based chemoresistance occurs constantly. Mitochondria, as the power factory in cancer cells, are involved in the process of chemoresistance. The dynamic balance of mitochondria is under the control of mitophagy. Stomatin-like protein 2 (STOML2) is located in the mitochondrial inner membrane and is highly expressed in cancer cells. In this study, using a tissue microarray (TMA), we found that high STOML2 expression was correlated with higher survival of patients with pancreatic cancer. Meanwhile, the proliferation and chemoresistance of pancreatic cancer cells could be retarded by STOML2. In addition, we found that STOML2 was positively related to mitochondrial mass and negatively related to mitophagy in pancreatic cancer cells. STOML2 stabilized PARL and further prevented gemcitabine-induced PINK1-dependent mitophagy. We also generated subcutaneous xenografts to verify the enhancement of gemcitabine therapy induced by STOML2. These findings suggested that STOML2 regulated the mitophagy process through the PARL/PINK1 pathway, thereby reducing the chemoresistance of pancreatic cancer. STOML2-overexpression targeted therapy might be helpful for gemcitabine sensitization in the future.





