-
Biotechnology
 Pathological Examination
Pathological ExaminationGenome Sequencing Services
Gene / protein chip
In situ hybridization detection
Tissue Chips Preparation
Paraffin-Embedded Tissue
Special Staining
Immunohistochemistry (IHC)
HE Staining
Cell ExperimentsCell Lines Culture & STR Identification Service
Primary Cell Preparation and Culture

site:
Home Page / Biotechnology / Pathological Examination

Biotechnology
-
Pathological Examination
-
Animal Experiment Platform
-
Cell Experiments
-
Cell Lines Culture & STR Identification Service
-
Primary Cell Preparation and Culture
-
Cell proliferation and activity detection
-
Transwell Migration/Invasion Assays
-
Cell Colony Formation Assay
-
Construction of stable transfection cell line
-
Double luciferase experiment
-
Flow Cytometry
-
Adhesion experiment
-
Scratch test
-
-
Immunological Tests
-
Molecular Biological Tests
clicks:37 2022-06-08 11:22:42In situ hybridization detectionOne. Experimental Principles
The basic principle of in situ hybridization technology is to use the complementary base sequence between the single strand of nucleic acid molecules to complement and pair the radioactive or non radioactive foreign nucleic acid (i.e. probe) with the DNA or RNA to be tested on the tissue, cell or chromosome to form a specific nucleic acid hybridization molecule, and display the position of the nucleic acid to be tested on the tissue, cell or chromosome by certain detection means.
Two. Application Introduction
Genomic in situ hybridization mainly uses the difference of DNA homology between species to block the genomic DNA of another species at an appropriate concentration and carry out in situ hybridization on the target chromosome.
Fluorescence in situ hybridization uses the fluorescent labeled nucleic acid fragment as the probe to hybridize with the DNA on the chromosome. The target DNA sequence of the signal DNA sequence on the chromosome or DNA micro section is detected by the fluorescence detection system (fluorescence microscope), and then the hybridization site is determined.
Multicolor fluorescence in situ hybridization is in situ hybridization with probes labeled with several different colors of fluorescein alone or mixed. It can detect multiple targets at the same time. Each target has different colors under fluorescence microscope and photos.
Three. Experimental Process
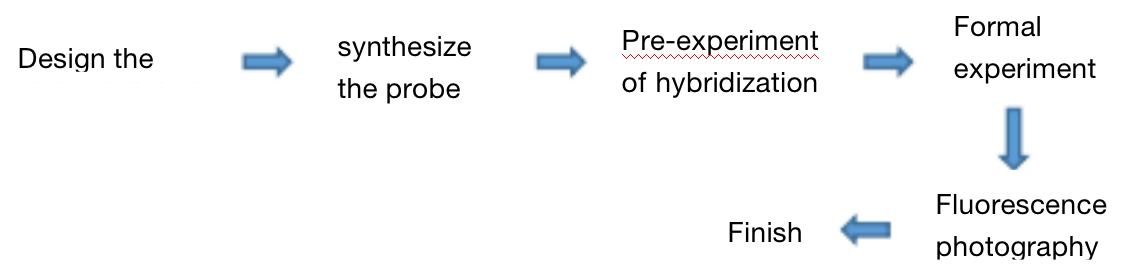
Four. Sample delivering requirements
Sample type
Sample requirements
Preservation conditions
Delivery conditions
Note
Fresh tissue
For the fresh tissue, it should be washed the blood away with PBS and preserve in -80°C immediately
At -80°C
With dry ice
All samples need to be uniquely marked and the markings are clearly identifiable
Regular fixed tissue
The volum of the regular tissue is within 2cm*2cm*0.5cm in general. Fixation is to keep the original shape of the tissue as much as possible and clean up the blood and excess parts; the volum of fixative fluid should be at least 10 times of the tissue, and the container should be big enough to hold the tissue without squeezing it. The type of fixative fluid and the fixed duration should be marked
At ambient temperature
At ambient temperature
Special fixed tissue
For tissue like testicle, eyeball, spinal cord, muscle, and so on: the correspondent special fixative fluid is recommended for fixation to ensure the fixing effect.
Paraffin embedded tissue
The tissue should be embedded in a standard paraffin embedding cassette, and paraffin wax should be evenly bonded to the tissue with no crack; the thickness of the wax block is determined by the number of slices required, and the effective thickness should be at least 0.1 cm. Provide the paraffin embedded tissue made within six months, otherwise the antigens of the paraffin may be lost and fail to detect the protein when performing immunohistochemistry.
At ambient temperature
At ambient temperature
Tissue section
It needs to pick up the section with anti-drop slides and mark the thickness of the section, and the temperature and duration of baking. The tissue should be near the lower third of the slice
At 4°C
With ice bag
Cell climbing sheet
Use the special cell climbing sheet for the orifice plate. After the cell climbing sheet is fixed, wash it with PBS for 2 to 3 times, store it in PBS, and seal the orifice plate with sealing film. Each climbing sheet shall have a unique mark on the orifice plate cover and electronic grouping information to prevent the mark from being blurred during transportation.
At ambient temperature
At ambient temperature
Plant example
Fix the fresh tissue with FAA and its degree of lignification is not high; for thinner blades, if parallel blade slicing is required, it is difficult to ensure complete cutting; the diameter of the rhizome should be larger than 1 mm.
At ambient temperature
At ambient temperature
Antibody
There should be clear marks to identify the antigens on the containers and the instructions should be attached. The amount of antigens should be larger than the experiment required.
For tissue sections and tissue chips, calculate the amount of antibody according to a section of 100 μl after dilution; for the round coverslips of the 24 well plate cell climbing slide, calculate the amount of antibody according to a section of 100 μl after dilution; for the round coverslips of the 12 well plate cell climbing slide, calculate the amount of antibody according to a section of 400 μl after dilution
At -20°C
With ice bag or dry ice
Five. Case Display 
Six. Common Problems
1. The length of RNA probe is preferably 50-150bp. The probe has entered the cell, with high hybridization rate and short hybridization time.
2. In situ hybridization, the probe concentration should exceed the concentration of the target sequence. The probe concentration must give the maximum signal-to-noise ratio of the experiment, because the background color grade is related to the probe concentration.
3. The hybridization temperature should be 20-30 ℃ lower than the melting or dissolution temperature. The time of hybridization reaction can be shortened with the increase of probe concentration. Too short hybridization time will lead to incomplete hybridization, and too long hybridization time will increase nonspecific coloring.
4. Poor tissue fixation or sucrose dehydration will make the tissue have more vacuoles.
Seven. Service Process
-
