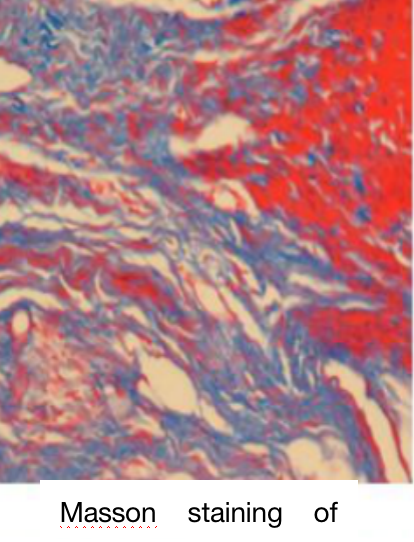
Taking Masson staining as an example
A. Experimental protocol
Masson trichrome staining, also known as Masson staining, is the most classic method of connective tissue staining. It is an authentic and classical technique of collagen fiber staining to show the fibers in tissues. The muscle fibers will be stained red, and the collagen fibers will be stained green or blue after Masson staining, distinguishing collagen fibers from muscle fibers. Collagen fibers show uniform pink in HE staining, which cannot be clearly distinguished from the surrounding stroma. However, Masson staining separates them clearly, and it is easy to observe the changes in collagen fibers in lesions.
B. Application
1) Organ sclerosing diseases observation: such as liver cirrhosis and myocardial scar, collagen staining makes the observation and diagnosis easier.
2) Identification between scar and amyloid: the former collagen fiber staining is positive, and the latter is negative.
3) Differentiation between the abnormal proliferation of bone fibers and bone fibrosis: it is easier to observe the disorder and crisscross of collagen fibers in the former and regularity in the latter by collagen fiber staining.
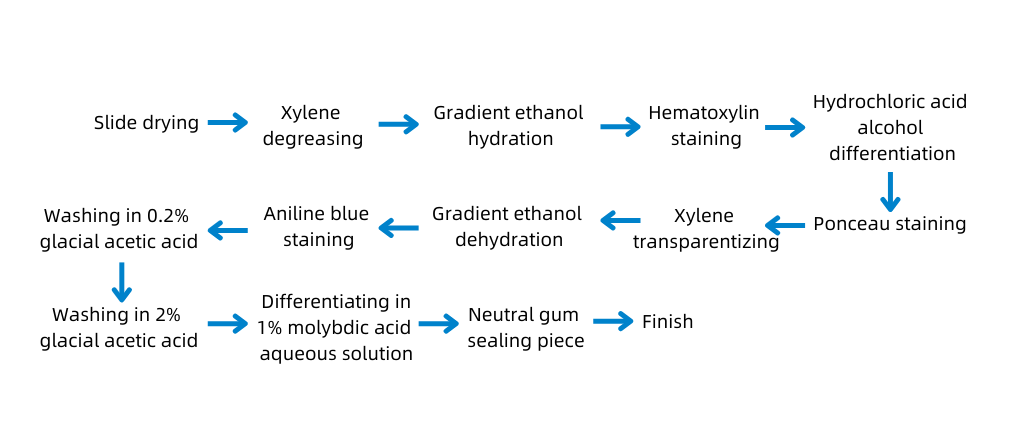
C. Experimental Protocol
D. Sample Submission Requirements
Sample type | Sample requirements | Preservation conditions | Submission conditions | Note |
Fresh tissue | The tissue is collected newly and freshly; after the blood is cleared away with PBS, the tissue is preserved at -80°C immediately | At -80°C | With dry ice | All samples need to be uniquely marked and the markings are identifiable |
Regular fixed tissue | The volume of the tissue is within 2cm*2cm*0.5cm in general. The tissue is fixed to keep its original shape as much as possible and clean up the blood and excess parts; the volume of fixative fluid should be at least 10 times the tissue, and the container should be big enough to hold the tissue without squeezing it. Label the fixative fluid type and the fixed duration. | At ambient temperature | At ambient temperature | |
Special fixed tissue | For tissue of testicle, eyeball, spinal cord, muscle, and so on: the correspondent special fixative fluid is recommended for fixation to ensure the fixing effect. | |||
Paraffin- embedded tissue | The tissue should be embedded with a standard embedding cassette and evenly combined without crack; the thickness of the wax block depends on the number of slices and the effective thickness should be over 0.1 cm. Provide the paraffin-embedded tissue made within six months. Otherwise, the antigens of the paraffin may be lost and fail to detect the protein when performing immunohistochemistry. | At ambient temperature | At ambient temperature | |
Tissue slices | It needs to pick up the section with anti-drop slides and label the thickness of the slice, the temperature, and the baking duration. The tissue should be near the lower third of the slice. | At 4°C | With ice bag | |
Cell climbing slices
| Use cell climbing film for the well plate. Wash the cell climbing film with PBS 2 to 3 times after fixation, store it in PBS, and seal the well plate with sealing film. Each well plate cover of the film should be marked uniquely, and electronic grouping information is required to prevent the mark from being blurred during transportation. | At ambient temperature | At ambient temperature | |
Plant example | Fix the fresh tissue with FAA and its degree of lignification should not be high; for thinner blades, if parallel blade slicing is required, it is difficult to ensure its integrity; the diameter of the rhizome should be larger than 1 mm. | At ambient temperature | At ambient temperature | |
Antibody | A clear mark to identify the antigen is required on the containers and the instructions should be attached. The amount of antigens should be more than that of the experiment required. For tissue slices and tissue chips, calculate the amount of antibody according to 100 μl/slice after dilution; for the round coverslips of the 24 well plate cell climbing slide, calculate the amount of antibody according to 200 μl/slice after dilution; for the round coverslips of the 12 well plate cell climbing slide, calculate the amount of antibody according to 400 μl/slice after diluted | At -20°C | With ice bag or dry ice |
E. Case Display
F. Common Problems
1. Detachment of slice when staining
① Cause: the tissue is broken or too hard;
Solution: use the anti-drop slides;
② Cause: the dehydration and clearing are insufficient ;
Solution: dehydrate, clear, and dip wax again;
③ Cause: the dehydration and clearing are excessive;
Solution: soak the cut surface in 1:1 alcohol and glycerin, and then slice again;
④ Cause: the slice is too thick;
Solution: slice evenly.
2. Uneven staining of sections
① Cause: improper sampling and fixing;
Solution: sample following the standard and fix completely;
② Cause: insufficient dehydration;
Solution: dehydrate, clear, and dip wax again.
3. Blurred slice after staining
① Excessive temperature of the wax;
② Over differentiation;
③ Incomplete dehydration and clearing.
G. Service Process