One. Experimental Principle
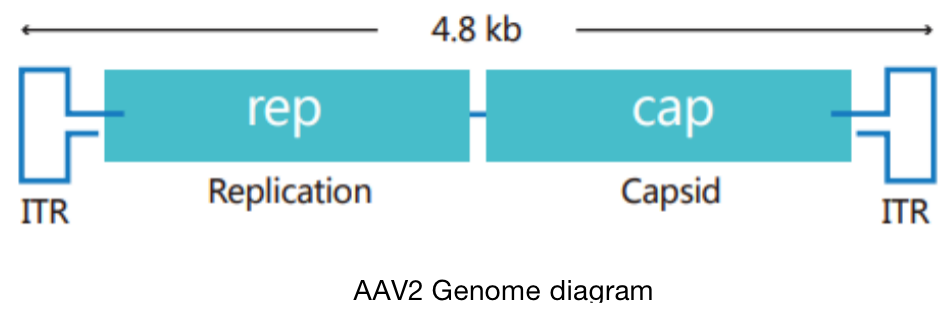
At present, the commonly used adenovirus vector is based on human adenovirus type 5 (Ad5), and its genome is 36Kb long linear double stranded DNA. Adenovirus can be endocytosed into cells through its own fiber and receptor binding on the cell surface, and then transferred from endosomes to cytoplasm and nucleus. With the help of cell transcription and translation machine, adenovirus replication and assembly can be started. A complete virus life cycle will cause cell death and release virus particles.
At present, the most commonly used adenovirus packaging systems are AdEasy and AdMax. Their common feature is that the target gene is first cloned into the shuttle vector, and then recombined into the large skeleton of adenovirus. Both systems have defects in adenovirus early transcription and replication genes E1 and E3( Δ E1, Δ E3), in which E3 gene is not necessary for virus production. Therefore, adenovirus packaging must depend on E1 expressing cell lines, such as HEK-293, hek-293a, etc.
Compared with AdEasy system, AdMax system is easy to operate and can obtain better virus titer. This operation instruction is based on AdMax system, which is composed of pHBAd series shuttle plasmid and adenovirus skeleton vector pBHGlox(delta)E1and 3Cre.
Two. Application Introduction
Adeno-associated virus (AAV) is the simplest single stranded DNA defective virus found at present, which needs auxiliary virus (usually adenovirus) to participate in replication.
Recombinant adenovirus is an adenovirus vector system for replication-deficient, which is widely used in gene therapy, basic life science research and other fields. Recombinant adenovirus has the following obvious advantages: it can infect almost all cell lines, primary cells and some tissues; The infection efficiency is up to 100%, which can comprehensively surpass other virus vector tools and liposome transfection; The carrying capacity of foreign genes is large (up to 8KB); It has unconformity genome, high titer and convenient operation. Therefore, recombinant adenovirus is one of the most potential gene delivery tools.
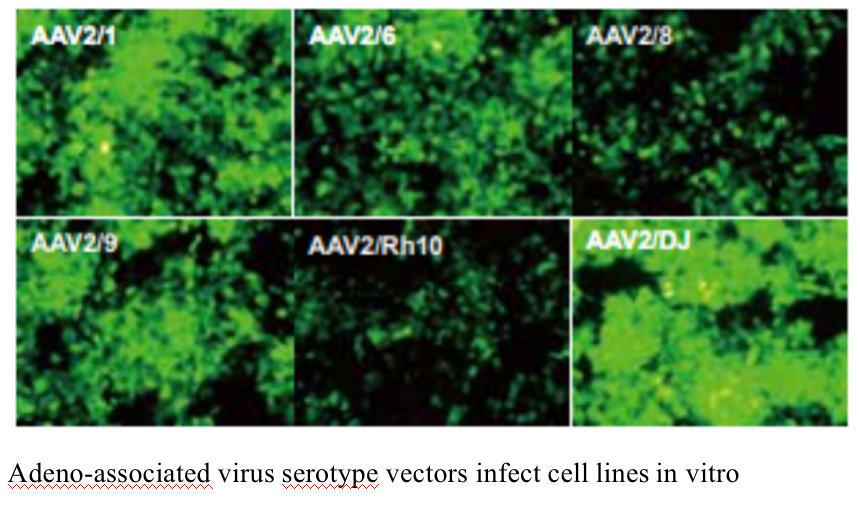
The experimental platform has a complete range of adeno-associated virus serotypes. The 10 stereotypes including AAV1、AAV2、AAV3、AAV4、AAV5、AAV6、AAV7、AAV8、AAV9、AAV-DJ are available. Double chain AAV (scaav) can also be packaged
Adeno-associated virus vectors include CMV、mCMV、CAG、PGK、RK1、EF1a、GFAP and other promoters; In addition, there are a variety of fluorescent labeled proteins such as EGFP, ZsGreen, tdTomato, mCherry and EYFP to choose from; At the same time, it can provide the packaging of inducible AAV virus, such as Tet-On system.
It can be used for: routine gene overexpression and interference with AAV customization; Tissue specific promoter overexpression AAV customization; Customization of tissue-specific DIO AAV (to be used in combination with tissue-specific CRE tool mouce); Aav-cas9 customization driven by conventional promoter and tissue-specific promoter; Aav-mrfp-gfp-lc3 autophagy flow monitoring; Various AAV mediated photogenetic and chemical genetic vectors and transformation services.
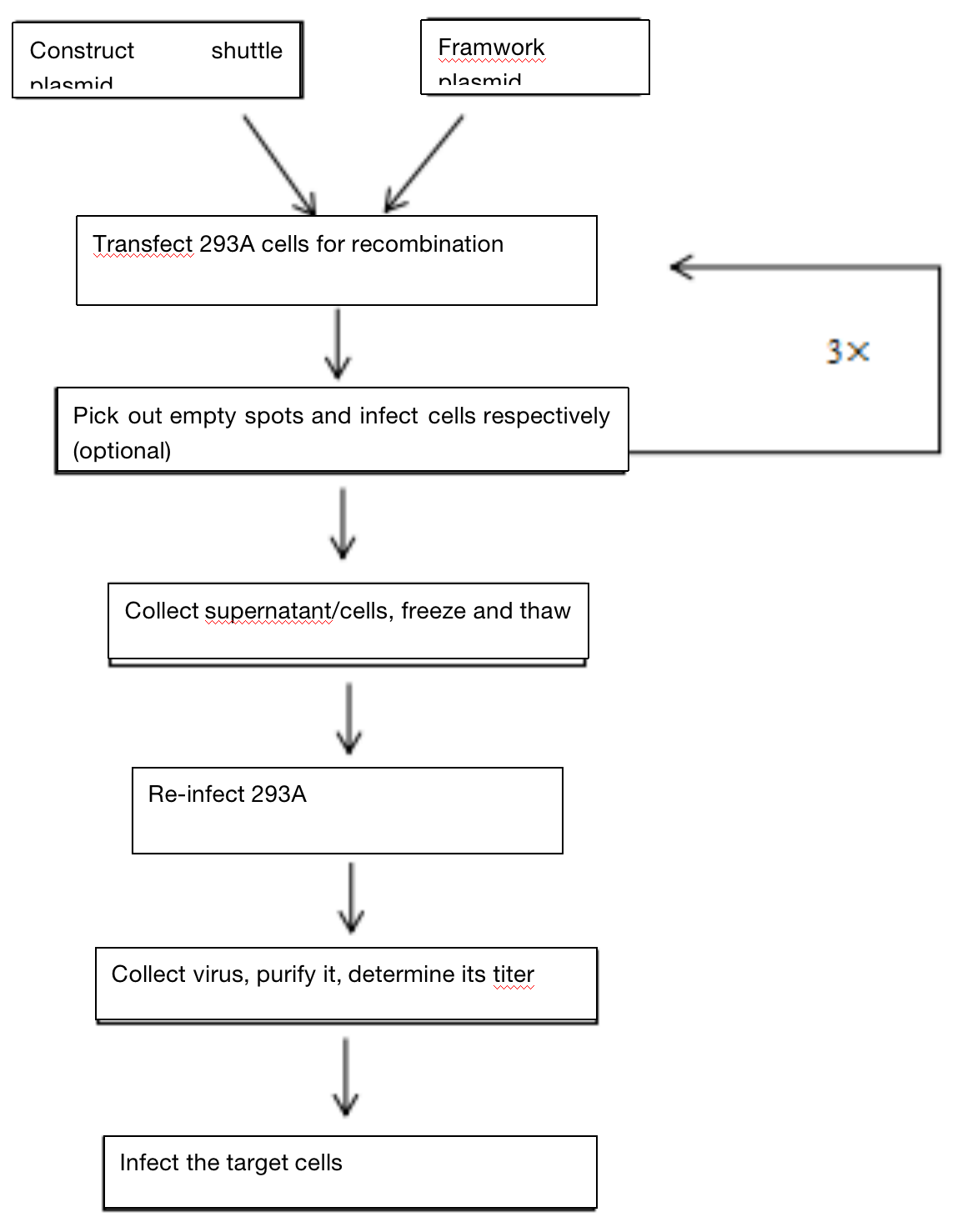
Three. Experimental Method
Four. Sample Delivering Requirements
Sample type | Sample requirements | Preservation conditions | Delivery conditions | Note |
Frozen cell | 1.Take the cells in logarithmic growth stage, digest and centrifuge to collect the cells, add the cryopreservation solution, blow and mix well, put it into the cryopreservation tube, mark the cell name/cell algebra, and the number of frozen cells is 1-5*106 / ml 2.After the start of the project, the cells will be resuscitated. Report the cell status 3 days after resuscitation, report the cell contamination 3 to 5 days later and report the mycoplasma contamination 5 tp 7 days later. 3.Cell details (name, culture medium and other culture conditions, etc. if it has been specially treated, it is necessary to inform and provide necessary information) | In liquid nitrogen | With dry ice | All samples need to be uniquely marked and the markings are clearly identifiable |
Resuscitated cell | 1.Use T25 culture bottle for transportation. When the confluence of cells reaches more than 60%, fill the bottle with culture medium, leave only bubbles of button size, seal the bottle with sealing film, mark the cell name/cell Algebra/ inoculation time/medium type, and transport in bottle stably. 2.Report the cell status 3 days after receiving, report the cell contamination 3 to 5 days later and report the mycoplasma contamination 5 tp 7 days later. 3.Cell details (name, culture medium and other culture conditions, etc. if it has been specially treated, it is necessary to inform and provide necessary information) | At ambient temperature | At ambient temperature | |
Plasmid | 1.High purity, no endotoxin, no protein, genomic DNA / RNA pollution, the ratio of plasmid A260:A280 is between 1.8-2.0 2.The concentration is not less than 0.5 μg/μl, the total amount is more than 100 μg, and there is no endotoxin treatment 3.Carrier details, including name, band, resistance, fluorescent labeling | At - 20℃ | With ice bag | |
Virus | 1. Lentivirus: the titer is not less than 1*108 TU/ml, the volume is about 200 μl, the preparation time is indicated, and there is no repeated freezing and thawing 2. Adenovirus: the titer is not less than 1*109 PFU/ml, the volume is about 200 μl, the preparation time is indicated, and there is no repeated freezing and thawing 3. To determine whether to express fluorescent label protein and its type, it is best to provide virus vector map | At - 80℃ | With dry ice |
Five. Case Display
Six. Common Problems
1. High purity low melting point agarose storage solution can be prepared in sterile PBS with a final concentration of 5%; Before use, completely melt it in boiling water bath, and then naturally cool it to 45 ℃. Dilute 5% agarose solution with complete medium preheated at 37 ℃ to the final concentration of 1.25%. After mixing, immediately add it to the cells that can remove the medium and cover it gently and evenly. Each well of the 6-well plate should be covered with 3 ml agarose/medium.
2. The cells must be in good growth condition before transfection.
3. When collecting cells, cell scraping is generally used instead of trypsin digestion. Centrifuge at 4 ℃ 500 ´ g for 10 min to remove most of the supernatant, leaving only 2 ml of supernatant to resuspend cell precipitation for - 80 ℃ (dry ice or liquid nitrogen can be used) freezing and thawing.
4. When freezing and thawing at 37 ℃, take out the virus as soon as it melts. Incubation at 37 ℃ for a long time will reduce the virus titer, and it can vibrate violently for 30s after complete melting. The freeze-thaw cycle usually lasts 2 ~ 4 times to obtain high titer virus.
At the end of cell freezing and thawing, the lysate can go through centrifugation at 4℃ 500×g for 10 min. And it can be frozen at - 20 ℃ or - 80 ℃ if it is not used immediately.
Seven. Service Process