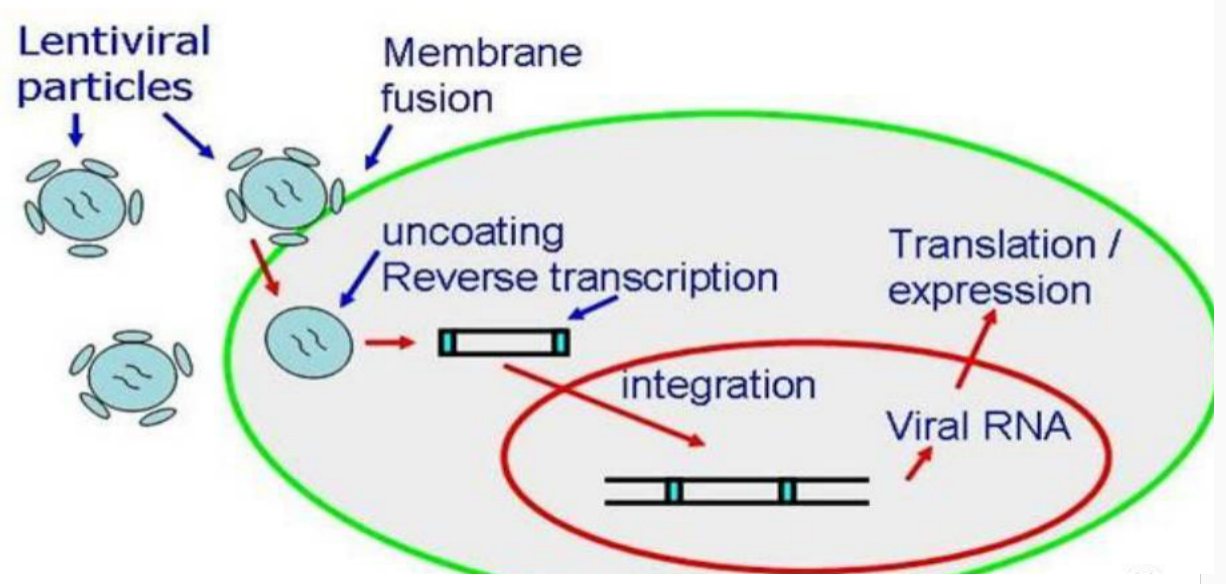
One. Experimental Principles
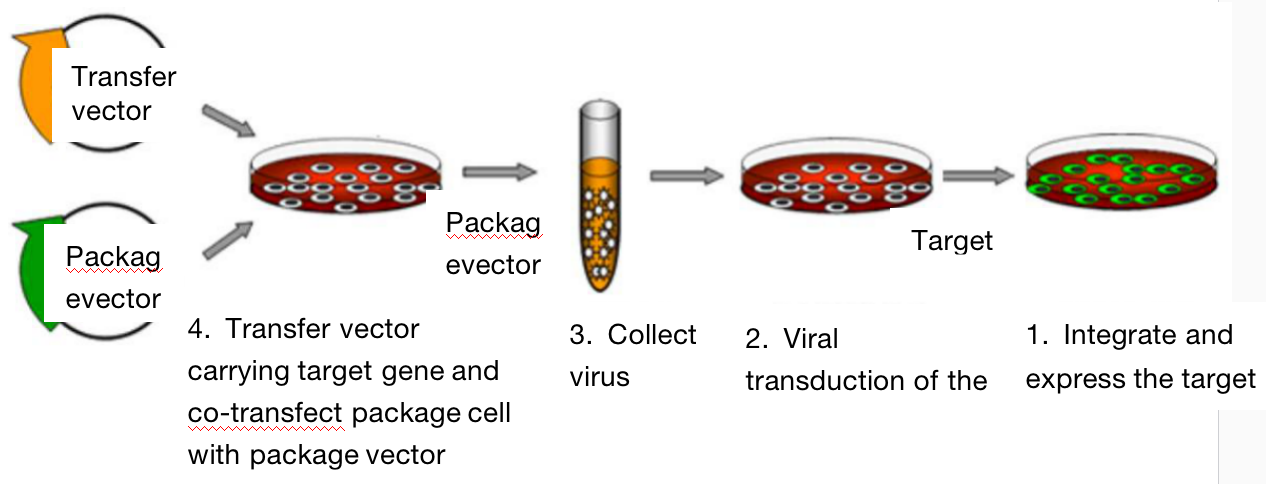
Lentivirus expression vector contains the genetic information needed for packaging, transfection and stable integration. Lentivirus packaging plasmid can provide all auxiliary proteins required by all transcribed, packed and recombined pseudovirus vectors. In order to produce high titer virus particles, it is necessary to co-transfect cells with expression vector and packaging plasmid at the same time and package the virus in cells. The packaged pseudovirus particles are secreted into the extracellular medium. The supernatant collected by centrifugation can be directly used for the infection of host cells. After entering the host cells, the target gene is reverse transcribed and integrated into the genome, so as to express effector molecules at a high level.
Two. Application Introduction
Lentivirus vector is a gene research vector based on HIV-1 virus. Different from retrovirus vector, it has the ability to infect both dividing and non dividing cells.
The lentivirus system of the experimental platform belongs to the third generation lentivirus system with high biosafety. It adopts VSVG envelope, has a wide host range and high lentivirus titer, and its virus titer can reach 10 ^ 9tu / ml.
At present, lentivirus vectors include CMV, MCMV, CAG, PGK, UBC, ef1a, GFAP, NSE and other promoters; In addition, there are a variety of fluorescent labeled proteins such as EGFP, ZsGreen, tdtomato, mCherry and EYFP to choose from; Antibiotic screening genes include puromycin, neomycin and hygromycin.
In cell experiments, for cells that are difficult or even impossible to be transfected by conventional methods, virus mediated method can greatly improve the gene transduction efficiency and achieve the purpose of high-efficiency expression of target genes.
Specifically, lentivirus packaging is mainly used in the following aspects:
1. For cells that are difficult to be transfected, such as primary cells, stem cells and undifferentiated cells, it can significantly improve the efficiency of target gene transduction, and the probability of target gene integration into the host cell genome is greatly increased, which vastly facilitates the research of RNAi, cDNA cloning and reporter gene;
2. Screen the stable transformation cell line;
3. Provide high-quality virus solution containing target gene for living animal model experiment.
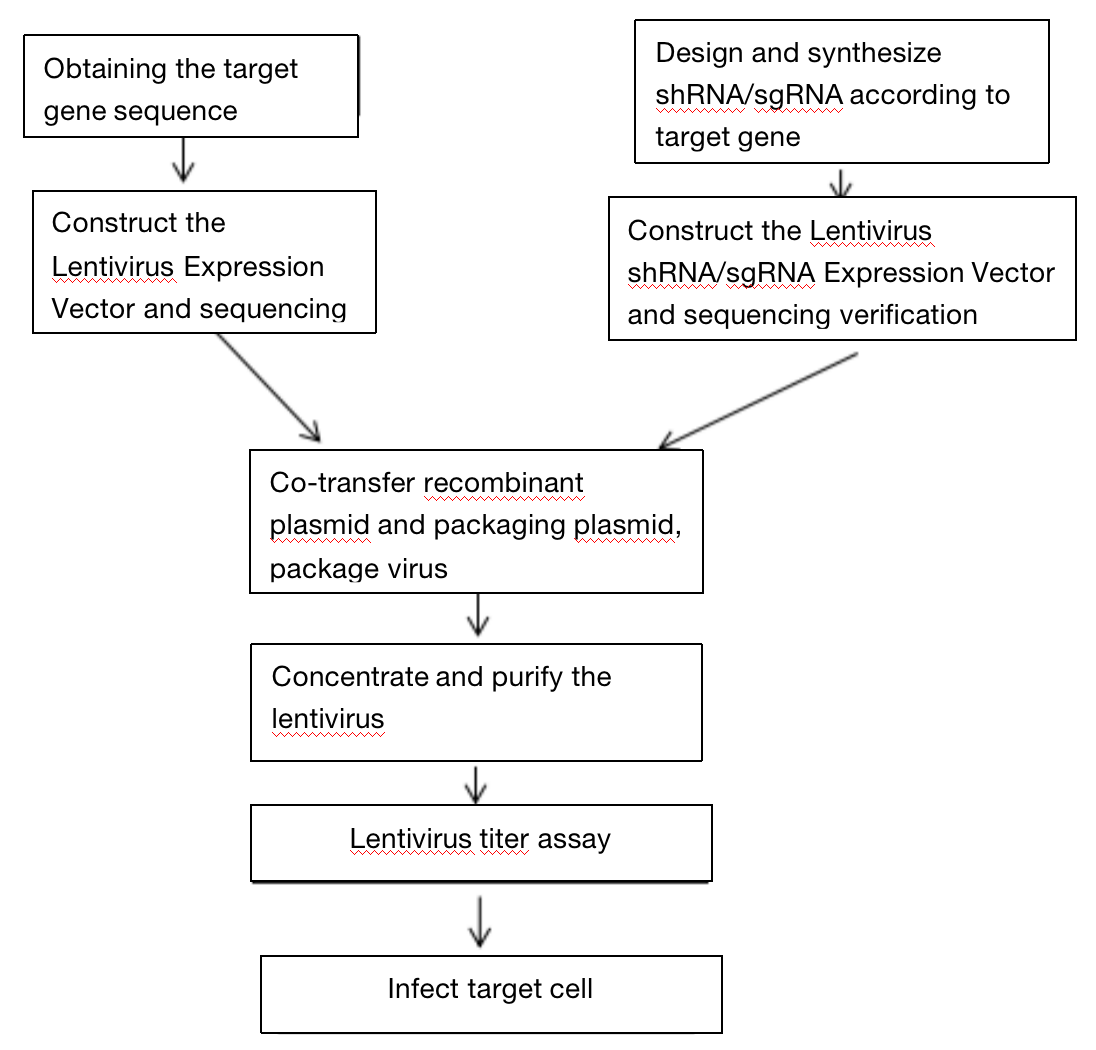
Three. Experimental Method
Four. Sample Delivering Requirements
Sample type | Sample requirements | Preservation conditions | Delivery conditions | Note |
Frozen cell | 1. Take the cells in logarithmic growth stage, digest and centrifuge to collect the cells, add the cryopreservation solution, blow and mix well, put it into the cryopreservation tube, mark the cell name/cell algebra, and the number of frozen cells is 1-5*106 / ml 2. After the start of the project, the cells will be resuscitated. Report the cell status 3 days after resuscitation, report the cell contamination 3 to 5 days later and report the mycoplasma contamination 5 tp 7 days later. 3. Cell details (name, culture medium and other culture conditions, etc. if it has been specially treated, it is necessary to inform and provide necessary information) | In liquid nitrogen | With dry ice | All samples need to be uniquely marked and the markings are clearly identifiable |
Resuscitated cell | 1. Use T25 culture bottle for transportation. When the confluence of cells reaches more than 60%, fill the bottle with culture medium, leave only bubbles of button size, seal the bottle with sealing film, mark the cell name/cell Algebra/ inoculation time/medium type, and transport in bottle stably. 2. Report the cell status 3 days after receiving, report the cell contamination 3 to 5 days later and report the mycoplasma contamination 5 tp 7 days later. 3. Cell details (name, culture medium and other culture conditions, etc. if it has been specially treated, it is necessary to inform and provide necessary information) | At ambient temperature | At ambient temperature | |
Plasmid | 1. High purity, no endotoxin, no protein, genomic DNA / RNA pollution, the ratio of plasmid A260:A280 is between 1.8-2.0 2. The concentration is not less than 0.5 μg/μl, the total amount is more than 100 μg, and there is no endotoxin treatment 3. Carrier details, including name, band, resistance, fluorescent labeling | At - 20℃ | With ice bag | |
Virus | 1. Lentivirus: the titer is not less than 1*108 TU/ml, the volume is about 200 μl, the preparation time is indicated, and there is no repeated freezing and thawing 2. Adenovirus: the titer is not less than 1*109 PFU/ml, the volume is about 200 μl, the preparation time is indicated, and there is no repeated freezing and thawing 3. To determine whether to express fluorescent label protein and its type, it is best to provide virus vector map | At - 80℃ | With dry ice |
Delivery standard:
Titer: > 108 TU/ml
Specification: 1ml
Complimentary negative control virus specification: 0.2ml (> 108 TU/ml)
Five. Case Display
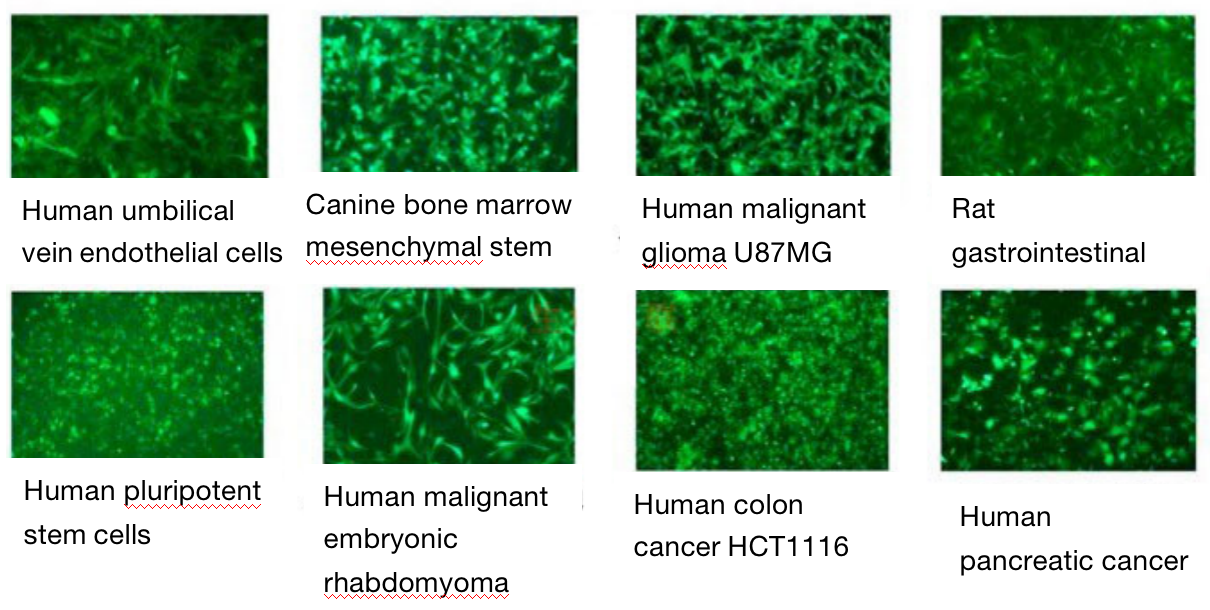
Six. Common Problems
1. In theory, lentivirus can package 5 ~ 7kbp fragments, but too large fragment packaging will affect the virus production efficiency, resulting in low virus titer and affect the effect of virus infection. Therefore, it is generally recommended to control the packaged fragment within 1.5kbp.
2. The viral vector is easy to mutate and lose. During the cloning process, the packaging signal mutation of plasmid or the loss of fragments of different sizes may occur, resulting in the failure to successfully package the virus. Therefore, it is suggested that the cloning vector containing the target gene (especially the insertion fragment greater than 1.5kbp) should be sequenced first to ensure that the key sequences are correct.
3. Packaging cells are mostly 293T or 293FT. The cells are required to grow vigorously and in good condition, so as to avoid over dense growth and too many in vitro passages.
4. The purity of plasmid is required to be high during transfection. It is best to be the super helical plasmid with endotoxin removed so as to improve the transfection efficiency. Calcium phosphate transfection method should be used often, which can not only save the cost, but also obtain high transfection efficiency (generally up to 80% ~ 95%).
5. Whether it is a three plasmid or four plasmid lentivirus system, the proportion of transfected plasmids is very important, which directly affects the virus production efficiency. The plasmids provided by different companies are different, so they cannot be generalized. They need to be adjusted and optimized according to the given proportion.
6. Before transfection, the cell density should be controlled at 50% ~ 60%. The fluid was changed 4 ~ 8h before transfection, and no fluid was changed after transfection. The addition of sodium butyrate (TPA) on the second day of transfection can significantly improve the virus yield, and then change the solution 8 hours later. The medium should be preheated to 37 ℃ before each liquid change, and the action should be gentle to prevent the cells from floating.
7. Generally, the virus particles harvested 48 hours after transfection are the most, but it is also related to the cell state, growth rate and pH value of culture medium at that time. The medium should be red when harvesting the virus. If it is too acidic and present yellow, the virus harvest rate will be reduced.
8. Sera from different manufacturers and batches have a great impact on the yield of virus and need to be screened.
9. Avoid repeated freezing and thawing of virus solution, otherwise the efficiency will be significantly reduced.
10. Generally, the virus solution can be stored at - 80 ℃ for 1 year, but it is recommended to retest the virus titer after half a year
Seven. Service Process