One. Experiment Principle
Flow cytometry (FCM) is a new and rapidly developing biomedical analysis technology. It is a new high-tech cell analysis technology integrating laser technology, photoelectric measurement technology, computer technology, hydrodynamics, cellular immunofluorescence chemistry technology and monoclonal antibody technology.
Flow cytometry enables cells or particles to flow in the liquid flow, pass through an incident beam one by one, and record the scattered light and various fluorescent signals with a high-sensitivity detector, so as to carry out multi-parameter analysis of particles, including particle shape and size, cell cycle, intracellular cytokines, bacterial surface antigen, cell DNA content, etc. The particles detected by FCM are generally active cells. A variety of biological information inside cells can be obtained by exciting the intensity and color of fluorescent substances labeled on cells and the intensity of scattered light by laser light source. In addition, FCM can also carry out cell sorting, which can sort according to some properties of the detected cells, and if the sorting process is carried out under sterile conditions, these cells can also be used for culture.
Two. Application Introduction
Medical application
1. HIV immunotyping, CD4 absolute counting
2. Immunotyping of leukemia and lymphoma
3. Cell cycle and ploidy analysis of tumor
4. Reticulocyte counting
5. Cross matching and immune status monitoring of cell transplantation
6. Stem cell counting
7. Residual leukemia cell examination
8. HLA-B27 examination
9. Platelet function and related diseases
Scientific research application
1. Application in immunology:
Combined with immunofluorescence method, flow cytometry can identify and count cells with different surface specific antigens, and study the competitiveness of various exogenous lectins binding to the cell surface.
2. Application in neurobiology research: Study on the characteristics of neural stem cells and neural progenitor cells; Identification and functional study of different types of nerve cells; Detection of cell activity, apoptosis and cell cycle.
3. Application in tumor research:
Flow cytometry can evaluate the effect of tumor chemotherapy and radiotherapy in experiment and clinic. Now the work of monitoring tumor treatment according to flow cytometry and cell dynamics data has been carried out in practical work.
4. Application in cell biology and molecular biology research:
Flow cytometry can be used to determine the percentage of cells in each phase of the cell cycle.
Flow cytometry can be used for multi parameter analysis, that is, to determine multiple properties of a cell at the same time.
5. Application in high-throughput monitoring and new drug research and development: Research on changes of cell function; Research on cell cycle and activity; New drug research and development; Research and development of culture medium, etc.
6. Marine biology: enrichment and analysis of bacteria, algae, phytoplankton, etc.
7. Microbiology: detection of bacteria and yeast; Fluorescent protein detection.
8. Biofuels
9. Genetic research: The chromosome frequency distribution can be obtained by measuring the chromosome DNA content by flow cytometry
Three. Experiment Method
Step 1: prepare single cell suspension (tissue sample)
Step 2: hemolysis (this step is not necessary for cell samples)
Step 3: centrifuge to remove supernatant
Step4: adjust cell density
Step 5: add the corresponding labeled antibody
Step 6: incubation
Step 7: centrifuge the supernatant after washing
Step8: on machine detection
Four. Samples Delivery Requirements
Sample Type | Sample Requirements | Delivery conditions |
Tissue | The isolated tissues are made into cell suspension and added into PBS medium | At ambient temperature |
Living cell | Sample amount: 1*10^6 cells/TEST, add into PBS medium | At ambient temperature |
Blood | Collect the blood in the anticoagulant blood collection vessel of EDTA heparin sodium. | At ambient temperature |
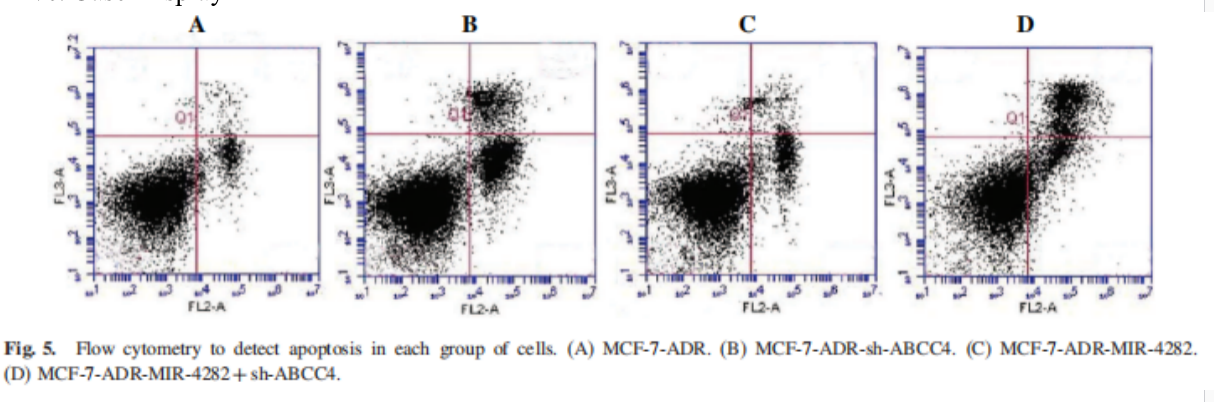
Five. Case Display
Six. Common Problems
1. The sample size to be tested should be at least 1 ml. The sample should be processed within 6h after collection. Frozen samples or hemolytic samples should not be used.
2. The cell activity should be good, otherwise nonspecific fluorescence staining is easy to occur.
3. Ensure that the cell concentration of the sample is 1*106 ml before being tested on the machine × 106 / ml, too low cell concentration will directly affect the test results.
4. In terms of operation, ensure that the flow cytometer is in the best state in the whole working process, and ensure the precision and accuracy of quantitative detection. Using standard samples to adjust the coefficient of variation of the instrument in the minimum range and the resolution in the best state can avoid the detection error caused by the change of instrument conditions in the measurement process.
5. On the scatter diagram of red fluorescence versus blue fluorescence, we can also see the migration track from apoptotic area to necrotic area, which may be due to the further degradation of DNA of apoptotic cells.
6. The incubation time between Heochst 33342 staining solution and cells should not be too long. Generally, it is better to control it within 20min. If it is too long, it may cause the emission spectrum of Heochst 33342 to migrate from blue light to red light, resulting in the change of the ratio of red fluorescence to blue fluorescence, thus affecting the judgment of the result.
Seven. Service Process