One. Experiment Principle
Matrigel is a matrix component extracted from mouse EHS sarcoma. It contains LN, type IV collagen, contactin and heparin sulfate polysaccharide. It is laid on the polycarbonate filter membrane without polyvinylpyrrolidone. It can reconstruct and form a membrane structure in DMEM medium. This membrane structure is very similar to that of natural matrix membrane.
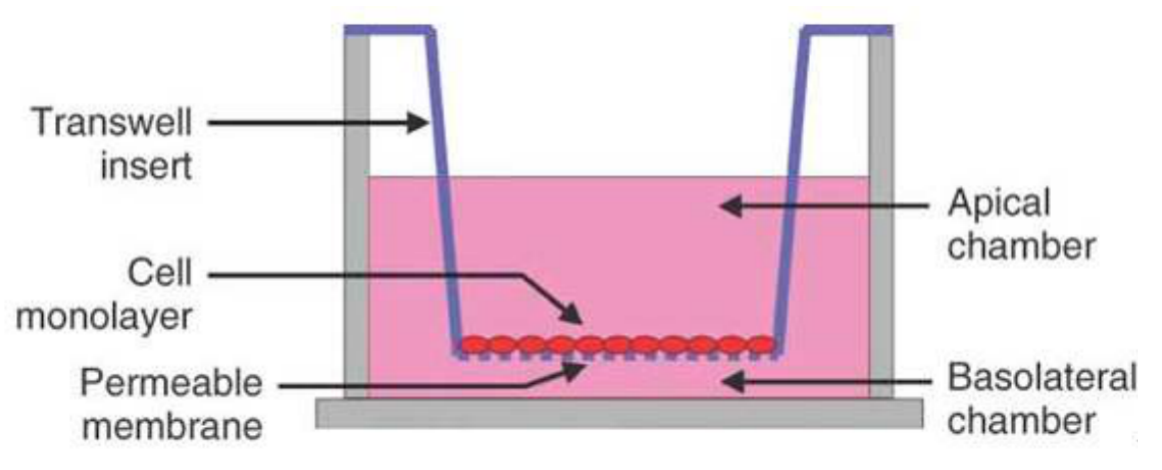
The pore size of the filter membrane is generally 8um, and the membrane pores are covered by Matrigel. Cells cannot pass through freely. They must secrete hydrolases and pass through the martrigel covered filter membrane through deformation movement, which is similar to the situation in vivo.
The filter membrane covered with martrigel is placed between the upper and lower chambers of Blind Well chamber or MICS chamber. The membrane paved with Martrigel is facing upward. Chemoattractants are added to the lower chamber, such as LN, FN or mouse 3T3 conditioned medium or human testicular epithelial fibroblast culture medium of a certain concentration. Suspended tumor cells are added to the upper chamber, and the invasive cells begin to penetrate the membrane under the induction of chemoattractants. The time of cell membrane penetration is related to the dosage of martrigel. 25ug martrigel is selected to pave the membrane, and the observation results after 16 hours are more appropriate. Most of the cells passing through the filter membrane adhere to the lower surface of the filter membrane. The cells on the upper surface can be wiped off with a cotton swab, and then the filter membrane is fixed with ethanol and stained with PE. The number of cells passing through martrigel is observed and counted under a light microscope.
In addition, the invasion analysis of reconstructed matrix membrane can also be carried out with Transwell cell. This method is to pave 30ug martrigel on the filter membrane of the basket upper cavity of Transwell cell, and observe the results 72 hours after adding cells. It is worth noting that a considerable number of cells passing through the filter membrane no longer adhere to the lower surface of the filter membrane, but fall off into the human lower cavity solution after the cells are cultured in the transwell cavity for 72 hours. Therefore, this part of cells should be taken into account when counting the number of cells passing through the matrix membrane. The ability of tumor cells to pass through the reconstructed stromal membrane shows a good correlation with its ability of invasion and metastasis in vivo. The reconstructed stromal membrane model can be used to screen anti-invasive drugs.
In the above analysis, if the 8um pore size filter membrane is directly placed between the upper and lower chambers of the invasion chamber without laying martrigel on the membrane, the cells pass through the filter membrane through deformation movement. This model is used to analyze the effect of cell movement ability and drugs on cell movement ability. In addition, adding LN or FN in the lower chamber or laying LN or FN on the lower surface of the filter membrane can analyze the effect of drugs on the chemotaxis or chemotaxis of tumor cells
Two. Application Introduction
Tumour Invasion Assay can be used to:
(1) Study the effects of various cytokines on the invasion and metastasis of malignant tumor cells;
(2) Research on some new drugs that inhibit angiogenesis;
(3) Study the mechanism of tumor cell invasion and metastasis.
Three. Experiment Method
Step 1: prepare conditioned medium
Step 2: coat basement membrane
Step 3: hydrate basement membrane
Step 4: inoculate cells
Step5: culture cells
Step6: fixation and dyeing
Step7: microscopic examination
Four. Samples Delivery Requirements
Sample type | Sample requirements | Preservation conditions | Delivery conditions | Note |
Frozen cell | 1. Take the cells in logarithmic growth stage, digest and centrifuge to collect the cells, add the cryopreservation solution, blow and mix well, put it into the cryopreservation tube, mark the cell name/cell algebra, and the number of frozen cells is 1-5*106 / ml 2. After the start of the project, the cells will be resuscitated. Report the cell status 3 days after resuscitation, report the cell contamination 3 to 5 days later and report the mycoplasma contamination 5 tp 7 days later. 3. Cell details (name, culture medium and other culture conditions, etc. if it has been specially treated, it is necessary to inform and provide necessary information) | In liquid nitrogen | With dry ice | All samples need to be uniquely marked and the markings are clearly identifiable |
Resuscitated cell | 1. Use T25 culture bottle for transportation. When the confluence of cells reaches more than 60%, fill the bottle with culture medium, leave only bubbles of button size, seal the bottle with sealing film, mark the cell name/cell Algebra/ inoculation time/medium type, and transport in bottle stably. 2. Report the cell status 3 days after receiving, report the cell contamination 3 to 5 days later and report the mycoplasma contamination 5 tp 7 days later. 3. Cell details (name, culture medium and other culture conditions, etc. if it has been specially treated, it is necessary to inform and provide necessary information) | At ambient temperature | At ambient temperature | |
Plasmid | 1. High purity, no endotoxin, no protein, genomic DNA / RNA pollution, the ratio of plasmid A260:A280 is between 1.8-2.0 2. The concentration is not less than 0.5 μg/μl, the total amount is more than 100 μg, and there is no endotoxin treatment 3. Carrier details, including name, band, resistance, fluorescent labeling | At - 20℃ | With ice bag | |
Virus | 1. Lentivirus: the titer is not less than 1*108 TU/ml, the volume is about 200 μl, the preparation time is indicated, and there is no repeated freezing and thawing 2. Adenovirus: the titer is not less than 1*109 PFU/ml, the volume is about 200 μl, the preparation time is indicated, and there is no repeated freezing and thawing 3. To determine whether to express fluorescent label protein and its type, it is best to provide virus vector map | At - 80℃ | With dry ice |
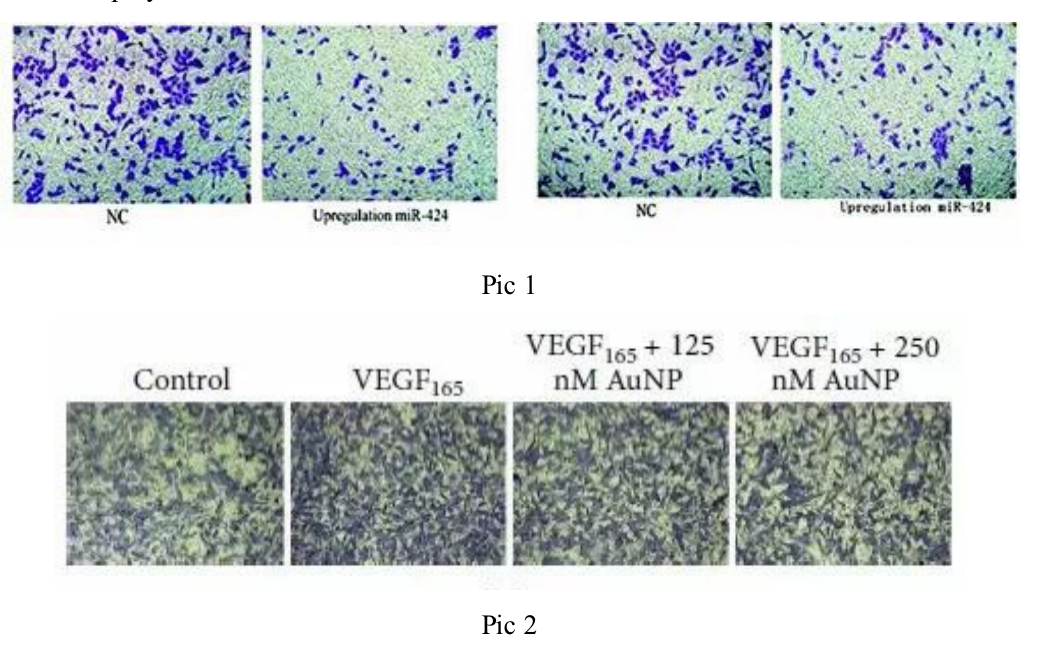
Five. Case Display
Six. Common Problems
1. 600ul culture medium is generally added into the lower chamber of the 24 well plate. Special attention is paid to that bubbles are often generated between the lower culture medium and the chamber. Once bubbles are generated, the chemotaxis of the lower culture medium will weaken or even disappear. Thus, pay special attention when seeding the plate. Once bubbles appear, lift the small space to remove the bubbles, and then put the chamber into the culture plate;
2. The inoculated cells should be in logarithmic growth stage and in good condition, and the cell count should be accurate;
3. The cells can be fixed and stained after migration and morphological expansion;
4. After staining, the upper layer of non-migrating cells can be gently wiped off with a cotton swab;
5. When taking photos, the same field of vision cannot be taken repeatedly. The small holes on the membrane are clear and the cell morphology is obvious. The background of all photos should be unified.
Seven. Service Process