A. Experimental principles
, qualitative and quantitative of antigens in histiocytes through the color reaction between enzyme-labeled antibody and substrate.
B. Application
Immunohistochemistry (IHC) has the advantages of strong specificity, high sensitivity and accurate localization.
1. It can be used in a variety of materials: various tissues (frozen tissue, formalin fixed tissue, paraffin embedded tissue); various cells (puncture, body fluid, pictures, blood cells, cultured cells, etc.).
2. It can be used in diagnosis: oncopathology, etc..
3. It can be used in scientific research: Clinical and basic medicine, especially morphology; In the research of molecular biology, immunohistochemical method is used to locate many macromolecules, and then its function is deeply studied.
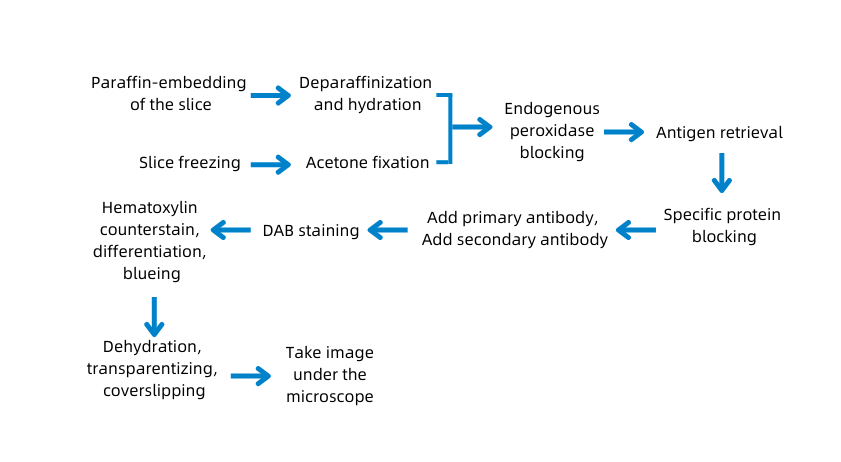
C. Experimental protocol
D. Sample Submission Requirements
Sample type | Sample requirements | Preservation conditions | Submission conditions | Note |
Fresh tissue | The tissue is collected newly and freshly; after the blood is cleared away with PBS, the tissue is preserved at -80°C immediately | At -80°C | With dry ice | All samples need to be uniquely marked and the markings are identifiable |
Regular fixed tissue | The volume of the tissue is within 2cm*2cm*0.5cm in general. The tissue is fixed to keep its original shape as much as possible and clean up the blood and excess parts; the volume of fixative fluid should be at least 10 times the tissue, and the container should be big enough to hold the tissue without squeezing it. Label the fixative fluid type and the fixed duration. | At ambient temperature | At ambient temperature | |
Special fixed tissue | For tissue of testicle, eyeball, spinal cord, muscle, and so on: the correspondent special fixative fluid is recommended for fixation to ensure the fixing effect. | |||
Paraffin- embedded tissue | The tissue should be embedded with a standard embedding cassette and evenly combined without crack; the thickness of the wax block depends on the number of slices and the effective thickness should be over 0.1 cm. Provide the paraffin-embedded tissue made within six months. Otherwise, the antigens of the paraffin may be lost and fail to detect the protein when performing immunohistochemistry. | At ambient temperature | At ambient temperature | |
Tissue slices | It needs to pick up the section with anti-drop slides and label the thickness of the slice, the temperature, and the baking duration. The tissue should be near the lower third of the slice. | At 4°C | With ice bag | |
Cell climbing slices
| Use cell climbing film for the well plate. Wash the cell climbing film with PBS 2 to 3 times after fixation, store it in PBS, and seal the well plate with sealing film. Each well plate cover of the film should be marked uniquely, and electronic grouping information is required to prevent the mark from being blurred during transportation. | At ambient temperature | At ambient temperature | |
Plant example | Fix the fresh tissue with FAA and its degree of lignification should not be high; for thinner blades, if parallel blade slicing is required, it is difficult to ensure its integrity; the diameter of the rhizome should be larger than 1 mm. | At ambient temperature | At ambient temperature | |
Antibody | A clear mark to identify the antigen is required on the containers and the instructions should be attached. The amount of antigens should be more than that of the experiment required. For tissue slices and tissue chips, calculate the amount of antibody according to 100 μl/slice after dilution; for the round coverslips of the 24 well plate cell climbing slide, calculate the amount of antibody according to 200 μl/slice after dilution; for the round coverslips of the 12 well plate cell climbing slide, calculate the amount of antibody according to 400 μl/slice after diluted | At -20°C | With ice bag or dry ice |
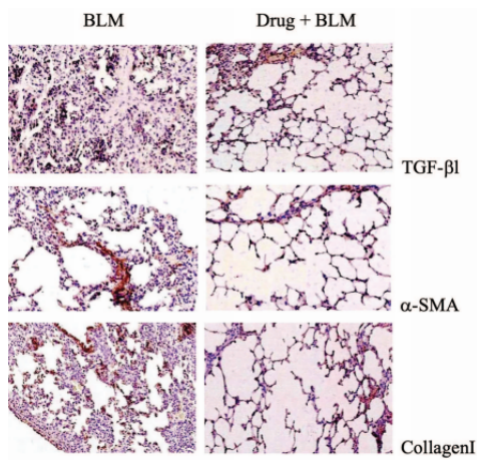
E. Case Display
F. Common Problems
(1)Control group or slice unstained
① Check whether some required reagents are ignored, including primary antibody, second antibody, third antibody, and substrate.
② Check whether all reagents are added in the correct order and incubated for enough time.
③ It is important to check whether the correct antibody is used and whether the detection system matches the primary antibody. For example, if the primary antibody is rabbit-derived, the second antibody must be anti-rabbit; if the primary antibody is the mouse IgM primary antibody, the second antibody must be goat/rabbit anti-mouse IgM (not IgG).
④ Check the dilution and dilute solution used by the antibody.
⑤ Check the validity period and storage conditions of antibodies, especially those labeled with enzyme or fluorescein. At present, antibodies from most reagent companies need to be stored at 4 ~ 8 ℃, and repeated freezing and thawing are forbidden. The reagents must not be stored in the same room with a volatile organic solvent, which will decrease the reagents’ titer.
⑥ Check the storage conditions of samples. Known positive samples are recommended as a positive control at the same time.
⑦ Check the chromogen/substrate solution. The simplest detection is adding a drop of enzyme-labeled antibody to the prepared substrate solution, the influence of the substrate can be excluded if the color changes as expected. It should be noted that some substrates should be used up within a certain time after being made into working fluid, otherwise they will become invalid.
⑧ Check whether the flushing solution matches the reagent. The pH value of the solution is very important. Thus, the solution matching the peroxidase substrate should contain no sodium azide.
⑨ Check whether the counterstaining solution and sealer match the used chromogen.
(2) Weak Positive
If the negative control is not stained and the positive control is weakly positive, the factors follow should take into account except for the above ones:
① The fixation of the slice. Improper fixation and high temperature during fixation will affect the quantity and quality of the detected antigen.
② Improper antigen retrieval. Due to the blocking effect of fixative on antigen during the production of paraffin section, heat-induced epitope retrieval, enzyme digestion, and the two methods simultaneously should be used. And it should refer to the instructions of the manufacturers and the specific conditions of the sample to determine the best way.
③ Excessive antibody dilution or improper incubation temperature and time. Generally, reagent manufacturers will give a range of reagent concentrations. However, as the samples come from various tissues and the treatment is different, the gradient test of the primary antibody should be carried out with reference to the concentration range to find the best one.
④ Too much left cleaning agent on the slice. The action of adding the antibody to the slice is equivalent to further diluting the antibody artificially.
⑤ Non-horizontal position of the slice when incubating. Slice not placed horizontally will lose its antibodies.
If the negative control does not react while the positive control reacts well, and the specimen is weakly positive, it is probably because the positive control uses different tissue or fixation method.
(3) Non-specific staining
① Check whether endogenous enzymes and biotin are effectively removed. Note that this step is non-essential to every tissue. However, for tissues rich in endogenous enzymes or biotin, such as the liver and kidney, endogenous enzymes and biotin should be considered. The solutions are as follows:
Inactivate alkaline phosphatase. The most common way is adding levamisole (24mg/ml) to the substrate solution and maintaining the pH value at 7.6 ~ 8.2 to remove most of the endogenous alkaline phosphatase. For the acid phosphatase that will interfere with staining, 50mmol/L tartaric acid can be used to inhibit it.
Saturate the endogenous biotin: avidin should be added in advance to saturate endogenous biotin to eliminate endogenous biotin so that there are no remaining binding sites. The specific process is to immerse the slice with 25ug/ml avidin solution for 15 minutes before ABC or SP staining. Then wash it with PBS for 15 minutes before staining.
② Check whether the blocking serum is correct. To reduce the non-specific background staining caused by charge adsorption, the non-immune serum of second antibody animals should be diluted into a 3% - 10% solution with PBS solution to incubate the slice. In that way, the adsorption sites are blocked. Other unrelated proteins, such as bovine serum albumin, are also often used. In addition, it is of great importance to avoid bleeding and necrotic areas when sampling. Recently, some domestic laboratories have replaced serum with 5% skimmed milk powder for antigen blocking, and the effect is satisfactory.
③ Check whether the antibody meets the test requirements. Impure antigen or similar epitope to the target antigen in the slice can lead to Non-specific staining. In that case, using a high-purity and high titer antibody or monoclonal antibody against a more specific epitope is the only solution.
④ Check whether the concentration of primary antibodies is excessive.
⑤ Check whether the cleaning is sufficient. The operating instruction should be strictly followed. As there is a certain amount of salt in the buffer, it is conducive to reducing the background staining. Therefore, the solution with 0.05mol/l Tris HCl and 0.15mol/l NaCl is suitable for most staining generally. Besides, Tween 20 can help for a better effect if added. For special stains, reagent companies will generally provide the formula of buffer solution.
⑥ Check whether DAB is used correctly. Different incubation times and preparation methods of DAB may result in different background colors. In order to ensure the correctness of the experimental results, please strictly follow the dropping sequence indicated in the instruction when using the DAB kit, and pay attention to correcting the pH value of distilled water. As insoluble particles are common when dissolving DAB powder, A complete removal is needed by filtering. Otherwise, they may deposit on the tissue of the slice and produce spotted coloring. In addition, the oxide produced by improper preservation of DAB can also deposit on the tissue. Therefore, DAB should be kept in a dry place away from light, prepared just before use, and added H2O2 before use. Also, the excessive incubation time will result in background staining.
⑦ Check whether the samples have dried up when staining. If so, it will cause non-specific staining of the edge.
⑧ Check whether there is cross-reaction between the second antibody and the endogenous tissue protein of the slice.
G. Service Process