One. Experimental Principle
ELISA is based on the solidification of antigen ot antibody and the enzyme marker os antigen or antibody. The antigen or antibody bound to the surface of the solid support still maintains its immunological activity, and the enzyme labeled antigen or antibody retains both its immunological activity and enzyme activity. During the determination, the tested sample (determining the antibody or antigen) reacts with the antigen or antibody on the surface of the solid support. The antigen-antibody complex formed on the solid support is separated from other substances in the liquid by washing. Then the enzyme labeled antigen or antibody is added and bound to the solid-phase support through reaction. At this time, the amount of enzyme on the solid phase is in a certain proportion to the amount of substance tested in the sample. After adding the substrate of enzyme reaction, the substrate is catalyzed by enzyme to become colored products. The amount of products is directly related to the amount of tested substances in the sample, so it can be analyzed qualitatively or quantitatively according to the color depth. Due to the high catalytic efficiency of the enzyme, it indirectly amplifies the results of the immune reaction and makes the determination method achieve high sensitivity.
At present, there are many enzyme-linked immunosorbent assay methods, which can be divided into:
Indirect method - used to determine antibodies
Double antibody sandwich method - mainly used for the determination of macromolecular antigens
Competitive inhibition method - mainly used for the determination of small molecule antigens
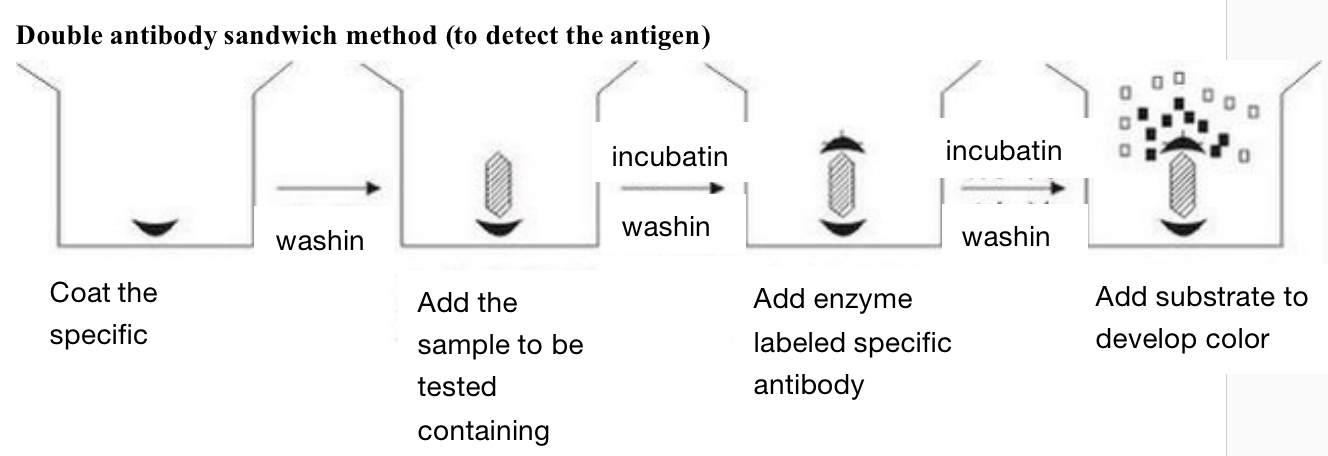
Firstly, the specific antibody is coated on the solid support. After washing, the sample to be tested containing antigen is added. If there is corresponding antigen in the sample to be tested, it can be combined with the specific antibody coated on the solid support. After incubation and washing, the enzyme labeled specific antibody can be added. After incubation and washing, the substrate is added to develop color for determination. The amount of substrate degradation is the amount of antigen to be measured.
The antigen to be tested by this method must have two sites that can bind to the antibody, because one end of it needs to interact with the antibody coated on the solid support, and the other end needs to interact with the enzyme labeled specific antibody. Therefore, it cannot be used for antigen determination such as hapten with molecular weight less than 5000. We use it in the determination of cholera enterotoxin and the determination of HBsAg and HBS.
Indirect method (to detect the antibody)
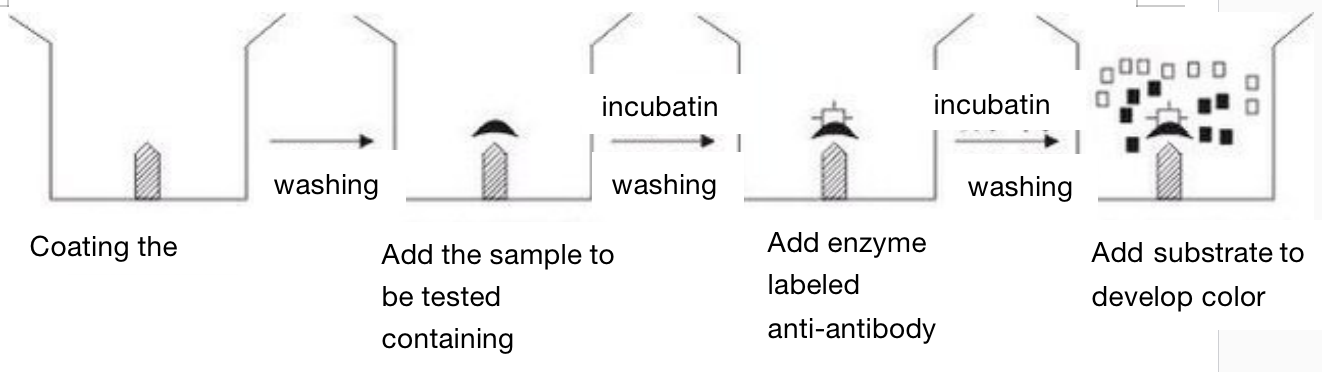
In the indirect method, the first step is to coat the solid support with the antigen, and the coated antigen must be soluble or at least very small particles. After washing, the sample containing the tested antibody is added. After incubation and washing, the enzyme labeled anti-antibody is added (for human samples, enzyme labeled anti human globulin IgG and IgM are added). After incubation and washing, the substrate is added for color development. The amount of substrate degradation is the amount of antibody to be measured, The results can be measured visually or quantitatively by spectrophotometer. After the solid support is coated with the same antigen, as long as an enzyme is used to label anti-human globulin, it can be used for serological diagnosis of various human infectious diseases, parasitic diseases and other diseases. If anti-human IgM is labeled with enzyme, it can be used for early diagnosis.
Competitive inhibition method (to detect the antigen)
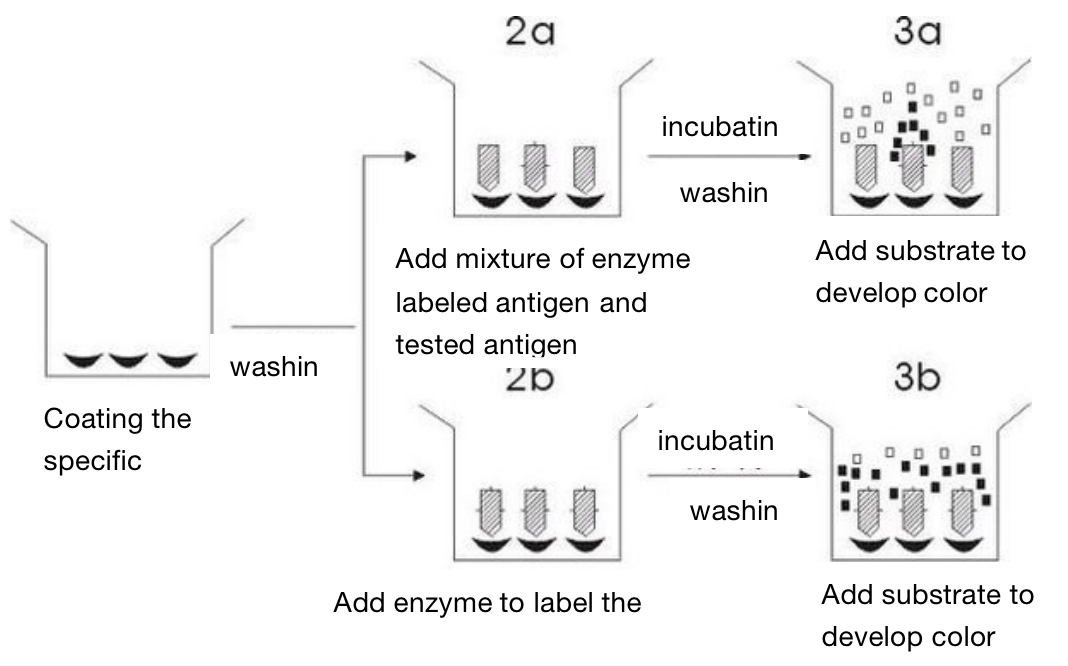
This method first adsorbs specific antibodies on the surface of solid support. The process of adsorbing antigens and antibodies on the surface ofsolid support is called coated, which can also be called sensitization. After washing, they are divided into two groups: one group is to add enzyme labeled antigen and tested antigen, while the other group only added enzyme labeled antigen, and then added substrate to develop color after incubation and washing. The difference of substrate degradation between the two groups is the amount of unknown antigen we want to determine.
The antigen determined by this method only needs one binding site. Therefore, this method is commonly used for the determination of small molecular antigens such as hormones and drugs. The advantage of this method is fast, because there is only one process for heating and washing . However, its disadvantage is that it needs a large amount of enzyme labeled antigen.
Two. Application Introduction
Enzyme linked immunosorbent assay (ELISA) can be applied to: (1) the location of various intracellular components by immunoenzyme staining. (2) the study of the synthesis of anti-enzyme antibody. (3) Show a trace of immunoprecipitation reaction. (4) Quantitative detection of antigen or antibody components in body fluid.
Three. Experimental Method
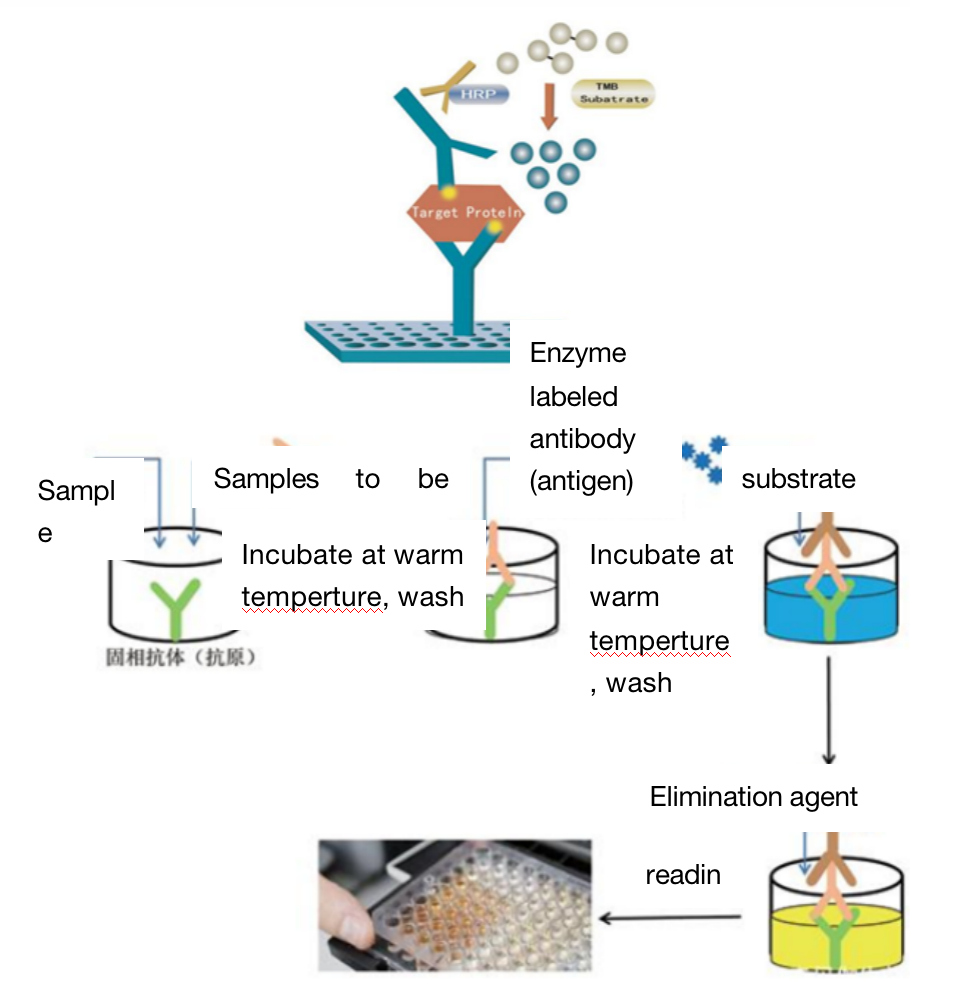
Four. Sample Delivering Requirements
Sample type | Sample requirements | Preservation conditions | Delivery conditions | Note |
Animal tissue | A single sample should be less than 0.1g, and installed in 2ml EP tube or frozen storage tube, and do not overdose it; Samples should be as fresh as possible. If protein or nucleic acid cannot be extracted immediately, they should be frozen at - 80 ℃ or lower after quick freezing with liquid nitrogen. Frozen samples should avoid repeated freezing and thawing to avoid degradation. | At - 80 ℃ | With dry ice | All samples need to be uniquely marked and the markings are clearly identifiable |
Seed sample | A single sample of seed that are shelled and fresh or stored in liquid nitrogen should be less than 0.2g | At - 80 ℃ | With dry ice | |
Adherent/Suspension cell | 1. Total RNA or protein: 10^5 cell/index, plasma/ nuclear RNA or protein:10^7 cell/index, mitochondrial RNA or protein: 2*10^7 cell/index.the samples should be as fresh as possible and should directly add Trizol (QPCR test) or frozen at - 80 ℃ after collected. 2. If the cells are in poor condition after treatment (dosing, transfection and infection), the sample collection volume should be increased as appropriate | At - 80 ℃ | With dry ice | |
Whole blood/serum samples | 5 to 10 ml of peripheral blood and 1 to 3 ml of bone marrow preserved with anticoagulant tubes. The leukocyte homogenate stored at -80 ℃ for no more than half a week is less than 400 μ L, and 400 μ L Trizol is added every 400 μ L | At - 80 ℃ | With dry ice | |
Paraffin embedded samples | The effective thickness of paraffin block embedded by standard paraffin embedding box should be thicker than 0.1cm; The thickness of each fresh FFPE tissue sections should be no more than 10 microns, and a surface area of no more than 250 mm^2, 2 to 8 piece in total. | At - 20℃ | With ice bag | |
Antibody | 1. Provide antibodies that meet the corresponding experimental requirements according to the sample species; 2. Send according to the requirements of the antibody manual, and try to avoid sub packaging; In case of sub packaging, ensure that the amount is sufficient for the experiment, and provide the antibody instruction; 3. The antibody tube storing the antibody should have a mark that can recognize the antibody, and the amount of antibody should be greater than the amount required for the experiment. | At - 20℃ | With ice bag | |
Primers | The primers should be dry powder and less than or equal to 1 OD. If pre experiment is conducted, at least two pairs of primers should be provided for each gene, and the primer synthesis sheet should be attached by the company, | At - 20℃ | With ice bag or at ambient temperature |
Five. Case Display
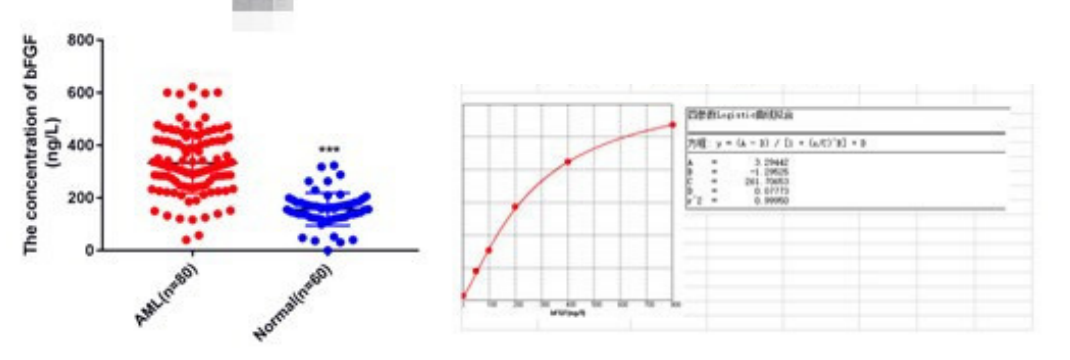
Six. Common Problems
1) Double antibody sandwich method (to detect antigen )
1. The basic condition for enzyme labeling determination is that soluble antigen or antibody is adsorbed on solid-phase carrier to become insoluble form. Many substances can be used as solid support, such as cellulose, cross-linked dextran, agarose beads, polypropylene, polystyrene, polyethylene and polyvinyl chloride. However, polystyrene or PVC micro reaction plates and plastic tubes are most commonly used in ELISA.
2. The antigen used for coating must be soluble, and it is required to be a high-quality and stable preparation with high purity and immunogenicity. Due to the limited competition between the pure antigen and the impurity in the solid phase, the sensitivity and specificity will be reduced. In addition, it should be considered that the preparation of coated antigen should not destroy its immunological activity.
3. Some antigens must be purified, such as tissue-cultured virus, chicken embryo culture and organs of virus inoculated animals. There are many non-antigen proteins in them, which must be removed. They are usually purified by gradient ultracentrifugation or affinity chromatography, and it can not be used without purification.
4. Coating the solid carrier with the optimal dilution antigen is not only economical, but also can overcome the prozone Phenomenon. It can be determined by chessboard titration, but the single titration method is more convenient, which is to conduct a series of dilution of the antigen, coat the micro reaction plate, incubate with a certain dilution of positive reference serum and negative reference serum, and then wash it, and add enzyme conjugate for reaction. After incubation and washing, add substrate solution for color development, and determine the OD value after reaction termination. Select the antigen dilution with OD value ≥ 1.0 as the optimal coating concentration. Generally, we should always choose the antigen concentration whose OD value is slightly greater than 1.0 rather than less than 1.0. The OD value of negative reference serum is required to be < 0.1-0.2. That is, the OD value of positive reference serum and negative reference serum is required to be significantly different.
5. Antigen coated solid support is related to time, temperature and pH in addition to concentration. When the temperature is high (such as 37 ℃, 45 ℃, or 56 ℃), the coating time can be shortened, and when the temperature is low, the coating time can be prolonged. However, for convenience, the antigen is usually coated at 4 ℃ overnight, which can make the antigen adsorption more complete and uniform. When polystyrene or polyethylene micro reaction plate or plastic tube is used as solid support, it is more appropriate to coat overnight at 4 ℃ under alkaline conditions (such as 0.1 mol / L, ph9.6 carbonate buffer diluting antigen). However, in some tests, such as coating with LPS or toxin protein, dilution with pH 7.2-7.4 PBS is appropriate. The concentration of coated specific protein is 1-10 ug/ml, while 5-20 ug/ml is more suitable for some pathogenic microbial antigens, but they should be titrated.
2) Indirect method (to detect antibody)
1. It should be noted that when separating serum, the blood is required to be coagulated and contracted at ambient temperature. It should not be coagulated in the refrigerator (4 ℃), otherwise most IgM and a small amount of IgG will lose activity.
2. In order to make the amount of antibody specifically bound as much as possible, the antigen coating the alcoves of the micro reaction plate should be slightly excessive.
3. During incubation or washing, part of the adsorbed antigen may be washed off the solid support. Thus, the protein other than the specific antibody added to the tested serum is likely to be adsorbed to the blank of the original coated alcoves, increase the background color and lead to false positive reaction.
4. Adding various protective inhibitors to diluents and detergents can inhibit the competitive adsorption of nonspecific proteins.
5. Tween-20 added in diluent and detergent is very important. It can not only eliminate the nonspecific background reaction, but also increase the sensitivity of positive samples, which may be related to the separation of nonspecific substances adsorbed by Tween-20. The detergent currently used is PBS in PH 7.4(containing 0.2 ‰ KCl) plus 0.05% Tween-20. If there is no Tween-20, it can also be replaced by Tween-40 or tween-60. However, Tween-80 has a high background and is not suitable for application.
3) Competitive inhibition method (to detect the antigen)
1. Repeatability of test (accuracy)
There are two methods to test the accuracy of the test: usually expressed by the coefficient of variation: 1. Several samples are determined simultaneously by ELISA to obtain CV%; 2. Several samples are measured by different operators at different times to obtain the coefficient of variation. CV is the ratio of the standard deviation of individual parameters to the mean.
S / x = CV, CV should be 10% lower and not more than 10 ~ 15%.
Regardless of visual inspection or spectrophotometer measurement, one dilution or a series of dilution can be measured. Generally, only one dilution is used for routine diagnosis to judge whether it is positive or negative for it is more convenient. Now it is recommended to use 1:200 dilution for serum diagnosis of infectious diseases. And a series of dilution determinations can be made for some require precise quantification.
There are several methods to record the results:
1. "+" or "-": all samples exceeding the specified OD value (e.g. OD, 0.4) are positive. The specified OD value is obtained from a large number of negative samples determined in advance, and the specified value is the upper limit of negative samples. Or it can take the average OD value of a group of negative samples plus 2 ~ 3 standard deviations as the positive threshold.
2. It is directly expressed by OD value, such as 0.4, 0.7 and 1.2, the greater the OD value, the stronger the positive reaction. However, this value is the result obtained under fixed experimental conditions, and there should be reference samples for control in each experiment.
3. Expressed by the end-point titer: continuously dilute the sample, and the titer of the sample is the one with positive reaction at the highest dilution (i.e. the OD value is still greater than the positive threshold).
4. Expressed by "ratio": calculate the ratio of the OD value of the tested sample to the OD value of a group of negative samples. If the ratio is 2 or 3 times of the negative sample, it is positive. If the ratio is less than 2.1 and greater than 1.5, it is suspicious, and < 1.5 is negative.
Determine the OD value of sample - blank OD value
Negative control OD value - blank OD value
Correct the "O" point with blank OD value during this determination.
5. Expressed in "unit": while determining the tested sample, determine a positive reference serum containing a known number of units. After ELISA detection with different dilutions, draw the standard curve with OD value as the ordinate and the number of units as the abscissa. The corresponding number of units can be found from the curve according to the OD value of the tested sample in the future, and then multiplied by the dilution multiple of the serum to obtain the number of units of the tested sample.
It is better not to color the negative reference serum during the test, otherwise it will bring difficulties to the judgment of the results. Especially during visual inspection, when the OD value of the positive sample is > 2.0, it should be properly diluted before determination.
6. Requirements for instruments
All instruments such as pipette, beaker and test tube should be cleaned, and the pipette and container used for enzyme binding and substrate must be separated. Reagents require standardization.
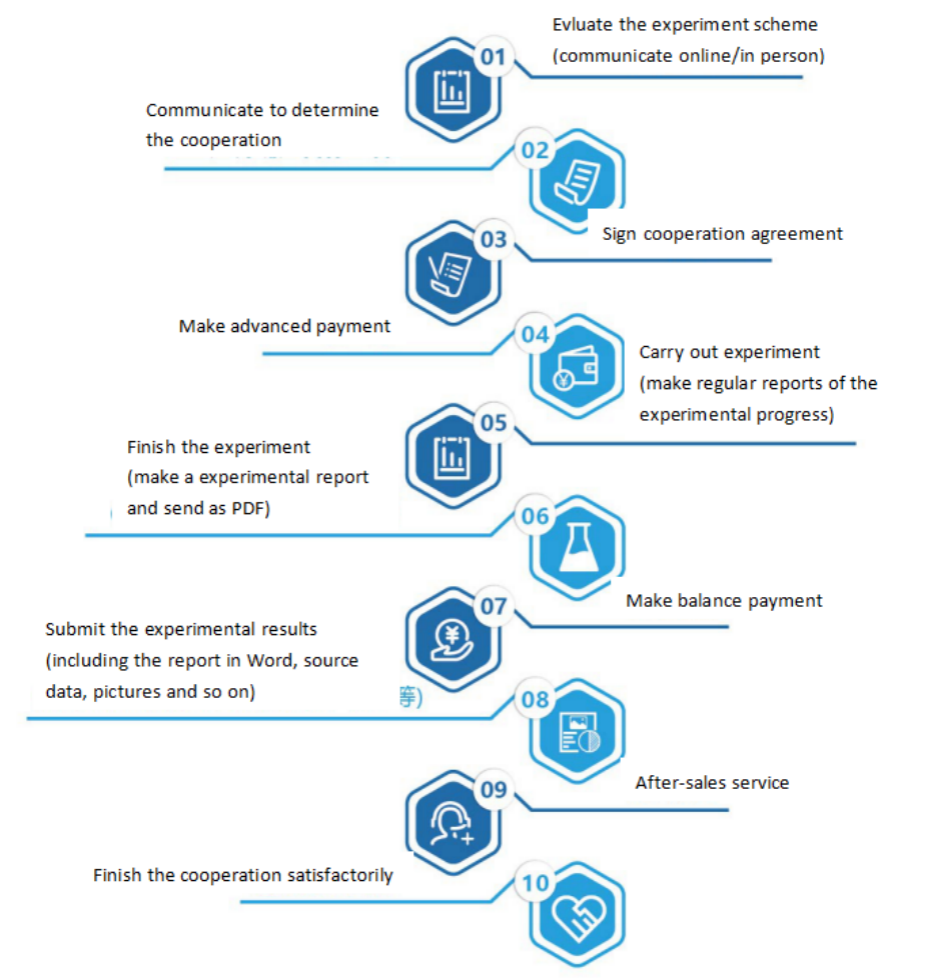
Seven. Service Process