One. Experimental Principle
Two. Application Introduction
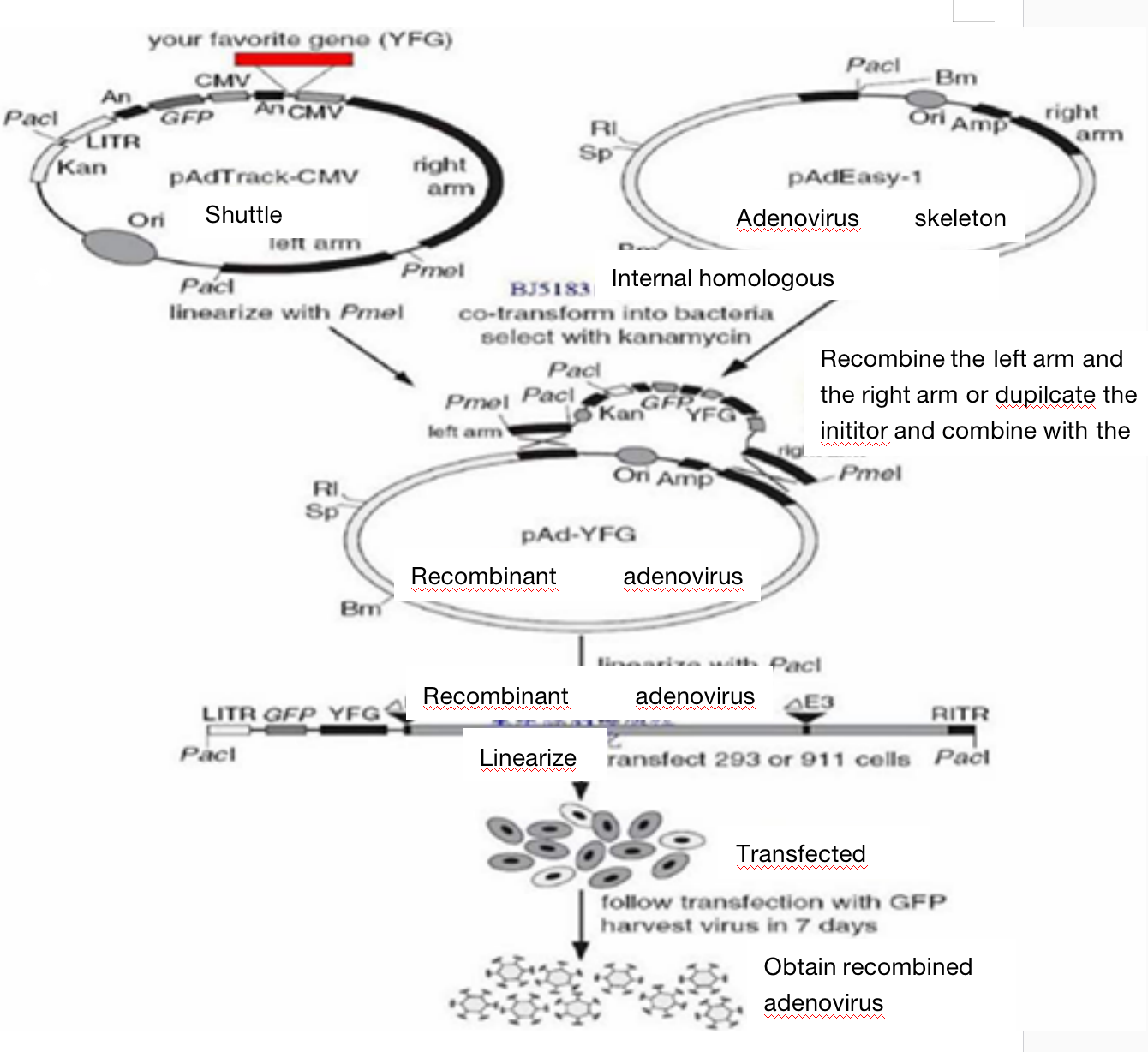
Adenovirus (adenovirus) is a virus particle with a diameter of 70 ~ 90 nm without envelope. It is a linear double stranded DNA molecule, containing about 35000 bp, and there are reverse repeat sequences with a length of about 100 bp at both ends.
The adenovirus of experimental platform is serotype 5 adenovirus, in which the E1 and E3 regions of the early expressed gene sequences are deleted. E1 is necessary for adenovirus replication. The deletion of E1 makes it unable to replicate itself. It can only rely on the trans complementarity provided by packaging cells such as HEK293 cells for replication and amplification, so as to ensure the safety of adenovirus. The protein expressed by E3 gene can resist the host's antiviral defense system, and the removal of E3 region can reduce the host's immune response in vivo.
Adenovirus packaging adopts the latest AdMax system. By using Cre- loxP recombinase, the adenovirus vector shuttle plasmid and skeleton plasmid (adenovirus genome plasmid) co transfected into HEK293 cells produce recombinant adenovirus under the action of recombinase. The virus titer is higher, and its virus titer can reach 1 × 10^12pfu/ml.
Adenovirus vectors include CMV, mCMV, CAG, PGK, UbC, EF1a and other promoters; In addition, there are a variety of fluorescent labeled proteins such as EGFP、ZsGreen、tdTomato、mCherry、EYFP and so on.
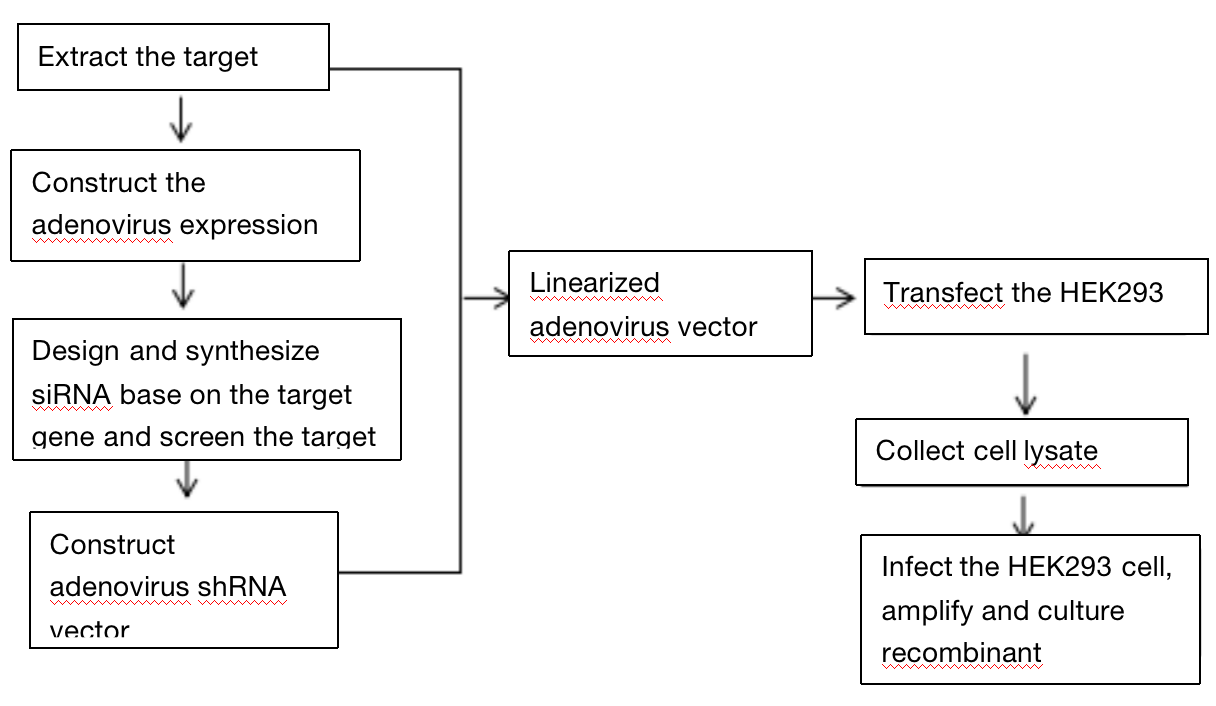
Three. Experimental Method
Four. Sample Delivering Requirements
Sample type | Sample requirements | Preservation conditions | Delivery conditions | Note |
Frozen cell | 1. Take the cells in logarithmic growth stage, digest and centrifuge to collect the cells, add the cryopreservation solution, blow and mix well, put it into the cryopreservation tube, mark the cell name/cell algebra, and the number of frozen cells is 1-5*106 / ml 2. After the start of the project, the cells will be resuscitated. Report the cell status 3 days after resuscitation, report the cell contamination 3 to 5 days later and report the mycoplasma contamination 5 tp 7 days later. 3. Cell details (name, culture medium and other culture conditions, etc. if it has been specially treated, it is necessary to inform and provide necessary information) | In liquid nitrogen | With dry ice | All samples need to be uniquely marked and the markings are clearly identifiable |
Resuscitated cell | 1. Use T25 culture bottle for transportation. When the confluence of cells reaches more than 60%, fill the bottle with culture medium, leave only bubbles of button size, seal the bottle with sealing film, mark the cell name/cell Algebra/ inoculation time/medium type, and transport in bottle stably. 2. Report the cell status 3 days after receiving, report the cell contamination 3 to 5 days later and report the mycoplasma contamination 5 tp 7 days later. 3. Cell details (name, culture medium and other culture conditions, etc. if it has been specially treated, it is necessary to inform and provide necessary information) | At ambient temperature | At ambient temperature | |
Plasmid | 1. High purity, no endotoxin, no protein, genomic DNA / RNA pollution, the ratio of plasmid A260:A280 is between 1.8-2.0 2. The concentration is not less than 0.5 μg/μl, the total amount is more than 100 μg, and there is no endotoxin treatment 3. Carrier details, including name, band, resistance, fluorescent labeling | At - 20℃ | With ice bag | |
Virus | 1. Lentivirus: the titer is not less than 1*108 TU/ml, the volume is about 200 μl, the preparation time is indicated, and there is no repeated freezing and thawing 2. Adenovirus: the titer is not less than 1*109 PFU/ml, the volume is about 200 μl, the preparation time is indicated, and there is no repeated freezing and thawing 3. To determine whether to express fluorescent label protein and its type, it is best to provide virus vector map | At - 80℃ | With dry ice |
Delivery standard:
Titer: > 108 TU/ml
Specification: 1ml
Complimentary negative control virus specification: 0.2ml (> 108 TU/ml)
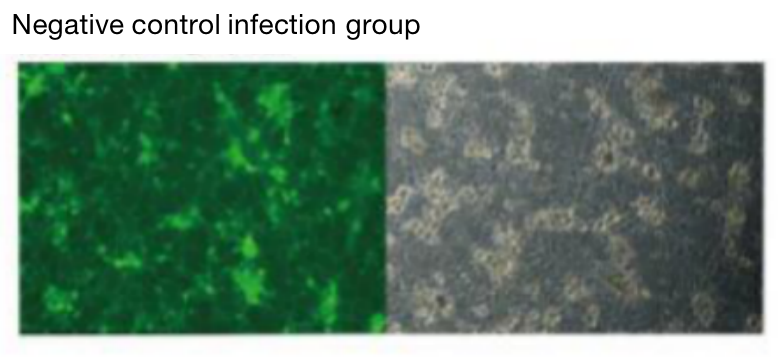
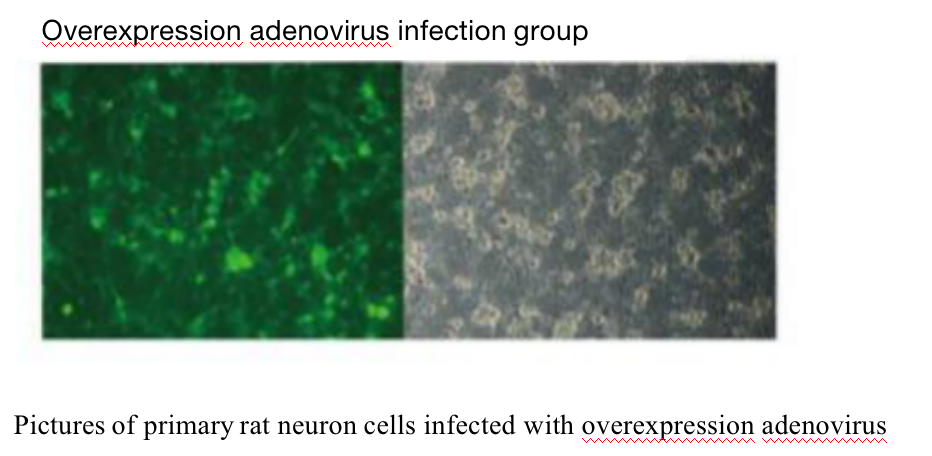
Five. Case Display
Negative control infection group
Six. Common Problems
Experimental problem | Cause | Recommended solution |
The yield of adenovirus with overexpressed gene is very low | Packing cells are in poor condition. | Please select 293 cells in good condition with earlier algebra |
The fragments of target gene are too long. | For genes above 3000bp, it is recommended not to increase the RES-EGFP markers, and increase the number of dishes in subsequent experiments | |
The yield of adenovirus amplification is very low. | The quality of virus stock solution is poor or the titer is too low. | Expand appropriately for one or two generations before large-scale amplification |
The adenovirus with EGFP fluorescence labeling had no fluorescence after infecting the cells. | Expression Cassettes is incorrect | Confirm whether the expression cassettes of foreign gene and EGFP is correct |
The cells are in poor condition | Make sure the cells are in good condition and explore appropriate MOI values | |
The adenovirus with EGFP fluorescence labeling had low fluorescence after infecting the cells. | The target genetic fragments are too long. | Too long fragment affects the fluorescence of co-expressed EGFP. It is suggested to observe the infection efficiency of GFP negative control adenovirus |
The cells are in poor condition | Make sure the cells are in good condition and explore appropriate MOI values | |
The overexpression of WB in cells infected with adenovirus with overexpressed gene was not obvious. The expression of the cell infected by the overexpressed gene adenovirus is not obvious through cell WB detection | Expression Cassettes is incorrect | Confirm whether the expression cassettes of adenovirus vector expressing gene is correct and there is no early termination |
The cells are in poor condition | Make sure the cells are in good condition and explore appropriate MOI values | |
The interference effect of gPCR detection is not good after adenovirus infected target cells | Unverified interference target | Confirm whether the target has been verified. If it is invalid, it is necessary to redesign the interference target |
The cells are in poor condition | Make sure the cells are in good condition and explore appropriate MOI values |
Seven. Service Process