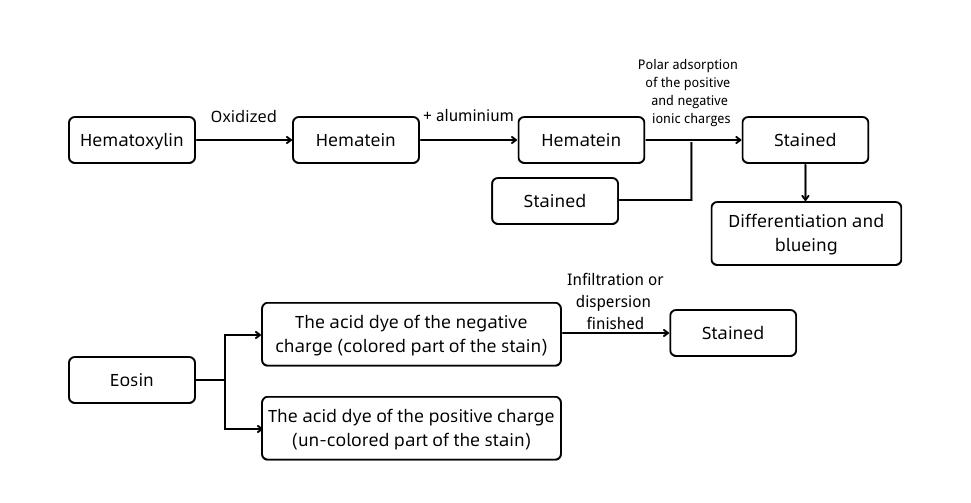
A. Experimental principle
Hematoxylin-eosin staining, also called HE staining for short, is one of the staining methods commonly used in the paraffin section.
Hematoxylin is a basic dye that can make the chromatin in the nucleus and nucleic acid in the cytoplasm purple-blue. Eosin is an acidic dye that can make the components in the cytoplasm and extracellular matrix red.
B. Application
HE staining is the most basic and widely used technique in the teaching and researching of histology, embryology, and pathology. It is also commonly used for the observation of tissue morphological structure.
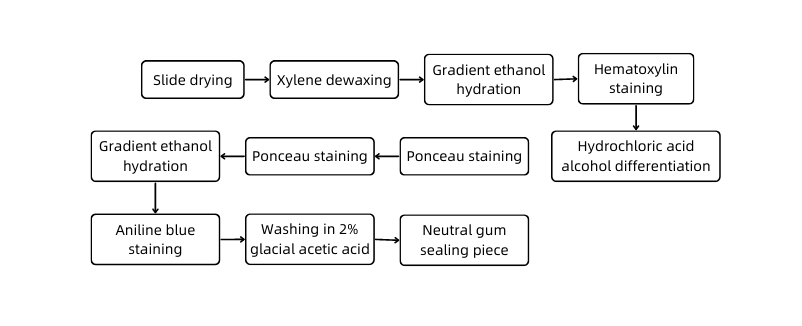
C. Experimental protocol
D. Sample Submission Requirements
Sample type | Sample requirements | Preservation conditions | Submission conditions | Note |
Fresh tissue | The tissue is collected newly and freshly; after the blood is cleared away with PBS, the tissue is preserved at -80°C immediately | At -80°C | With dry ice | All samples need to be uniquely marked and the markings are identifiable |
Regular tissue | The volume of the tissue is within 2cm*2cm*0.5cm in general. The tissue is fixed to keep its original shape as much as possible and clean up the blood and excess parts; the volume of fixative fluid should be at least 10 times the tissue, and the container should be big enough to hold the tissue without squeezing it. Label the fixative fluid type and the fixed duration. | At ambient temperature | At ambient temperature | |
Special tissue | For tissue of testicle, eyeball, spinal cord, muscle, and so on: the correspondent special fixative fluid is recommended for fixation to ensure the fixing effect. | |||
Paraffin- embedded tissue | The tissue should be embedded with a standard embedding cassette and evenly combined without crack; the thickness of the wax block depends on the number of slices and the effective thickness should be over 0.1 cm. Provide the paraffin-embedded tissue made within six months. Otherwise, the antigens of the paraffin may be lost and fail to detect the protein when performing immunohistochemistry. | At ambient temperature | At ambient temperature | |
Tissue slice | It needs to pick up the section with anti-drop slides and label the thickness of the slice, the temperature, and the baking duration. The tissue should be near the lower third of the slice. | At 4°C | With ice bag | |
Cell climbing slice
| Use cell climbing film for the well plate. Wash the cell climbing film with PBS 2 to 3 times after fixation, store it in PBS, and seal the well plate with sealing film. Each well plate cover of the film should be marked uniquely, and electronic grouping information is required to prevent the mark from being blurred during transportation. | At ambient temperature | At ambient temperature | |
Plant sample | Fix the fresh tissue with FAA and its degree of lignification should not be high; for thinner blades, if parallel blade slicing is required, it is difficult to ensure its integrity; the diameter of the rhizome should be larger than 1 mm. | At ambient temperature | At ambient temperature | |
Antibody | A clear mark to identify the antigen is required on the containers and the instructions should be attached. The amount of antigens should be more than that of the experiment required. For tissue slices and tissue chips, calculate the amount of antibody according to 100 μl/slice after dilution; for the round coverslips of the 24 well plate cell climbing slide, calculate the amount of antibody according to 200 μl/slice after dilution; for the round coverslips of the 12 well plate cell climbing slide, calculate the amount of antibody according to 400 μl/slice after diluted | At -20°C | With ice bag or dry ice |
E. Examples
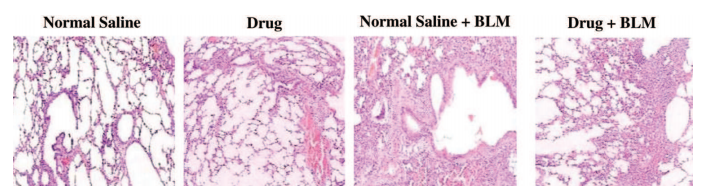
F. Common problems
1. Uneven staining
1.1 Improper tissue slicing and fixing
If the tissue is sliced too large or too thick, it cannot be fixed completely. And after staining, the fixed peripheral tissue can be colored easily while the unfixed part in the middle is blurry, resulting in uneven staining.
A solution to this problem is to fix the slices with 4% neutral formaldehyde in time, and the fixative fluid should be four times the volume of the samples. Large samples should be cut off before fixation.
1.2 Uneven thickness or transverse striations
A notched slicer may lead to fracture, fragmentation, and incompleteness. And if the inclination angle of the slicer is too large, the slices will roll up and cannot be linked together; if it is too small, the slicer will wrinkle. Besides, vibration due to loose screws will lead to uneven thickness of slices, and uneven force of technicians' shaking of the slicer head will cause transverse striations. Thus, it needs to check whether the slicer nut is tightened when slicing or whether the slicing angle is proper.
1.3 Improper dehydrating, clearing and waxing
(1) The concentrations of ethanol used for tissue dehydration are substandard, resulting in incomplete tissue dehydration.
(2) The tissue will be fragile if it is in the xylene for too long. And it is unfavorable for a good slice.
(3) If the wax temperature is too high, the tissue will become brittle, which is also unfavorable for slice staining.
1.4 Improper staining
(1) The paraffin removal of tissue slices is not complete. That is because the xylene is kept too long, the anhydrous ethanol is impure or kept too long, or the ambient temperature is low. In that case, the slices cannot be soaked entirely with the paraffin, causing coloring failure or insufficient coloring.
(2) If there are bubbles on the tissue slices when picking up, the tissues covered by the bubbles may be stained unevenly.
2. Blurred slices after staining
2.1 Slicing
The reasons for blurred slices after staining can be that the tissue sample is autolyzed, the tissue is not fixed in time after slicing, or the concentration of fixative fluid is insufficient. In addition, drying the sample before fixation will probably lead to gray staining after staining, which is typically irreversible.
2.2 Fixing
(1) The fixative solution can mordant the tissue to some extent. As it is able to combine with both protein and dyes, it can strengthen the coloring, and at the same time precipitate and coagulate the intracellular components, so that the intracellular components produce different refractive indexes causing optical differences. Therefore, poor fixation is the main reason for blurred slices after staining.
(2) Improper handling of lymph nodes in the daily making of pathological slides causes the phenomenon of graying of the tissue after staining. That is due to the dense cells and complex structure that make it difficult for the fixative to penetrate deep into the tissue, causing poor fixation in the center of the tissue and the phenomenon of complete graying. The solution is that treat the slices with a 1:1 anhydrous ethanol-ether mixture for 5-10 minutes, wash with ethanol, wash with water, and make supplemented mordant with 3% ferric ammonium sulfate solution for 5 minutes.
(3) Some of the submitted samples are fixed with ethanol. At ambient temperature, the albumin and globulin of ethanol are no longer soluble in water, while the nucleoprotein is soluble in water. Therefore, the slices fixed with ethanol have poor staining and cytoplasmic staining. If the slice is blurred, the remedy is to fix the sample again with 4% neutral formaldehyde.
3. Others
3.1 Temperature
Too high baking temperature after embedding or slicing will change the properties of protein and possibly lead to dye-exclusion, resulting in blurred slices after staining.
3.2 Dyes
The dyes have poor quality or are prepared improperly. For example, the hematoxylin cannot produce hematoxylin trioxide after heating to over-oxidation. Hence, no further generation of hematoxylin tetraoxide results in the incapacitation of the staining solution. Or the hematoxylin has been used for too long, resulting in poor staining and blurred slices.
3.3 Light staining of nucleus due to over differentiation
3.4 Dehydration
Incomplete clearing: if the slice in xylene generates white smoke, it demonstrates insufficient ethanol dehydration. A re-dehydration is urgent in that case. Otherwise, the tissue structure will be unclear under the microscope.
3.5 Sealing and fixing
(1) Avoid the breath from the mouth and nose of the operator from contacting the tissue sealer for the exhaled gas contains water;
(2) Move quickly in the humid environment to prevent water in the air from entering the sealer and affecting the quality.
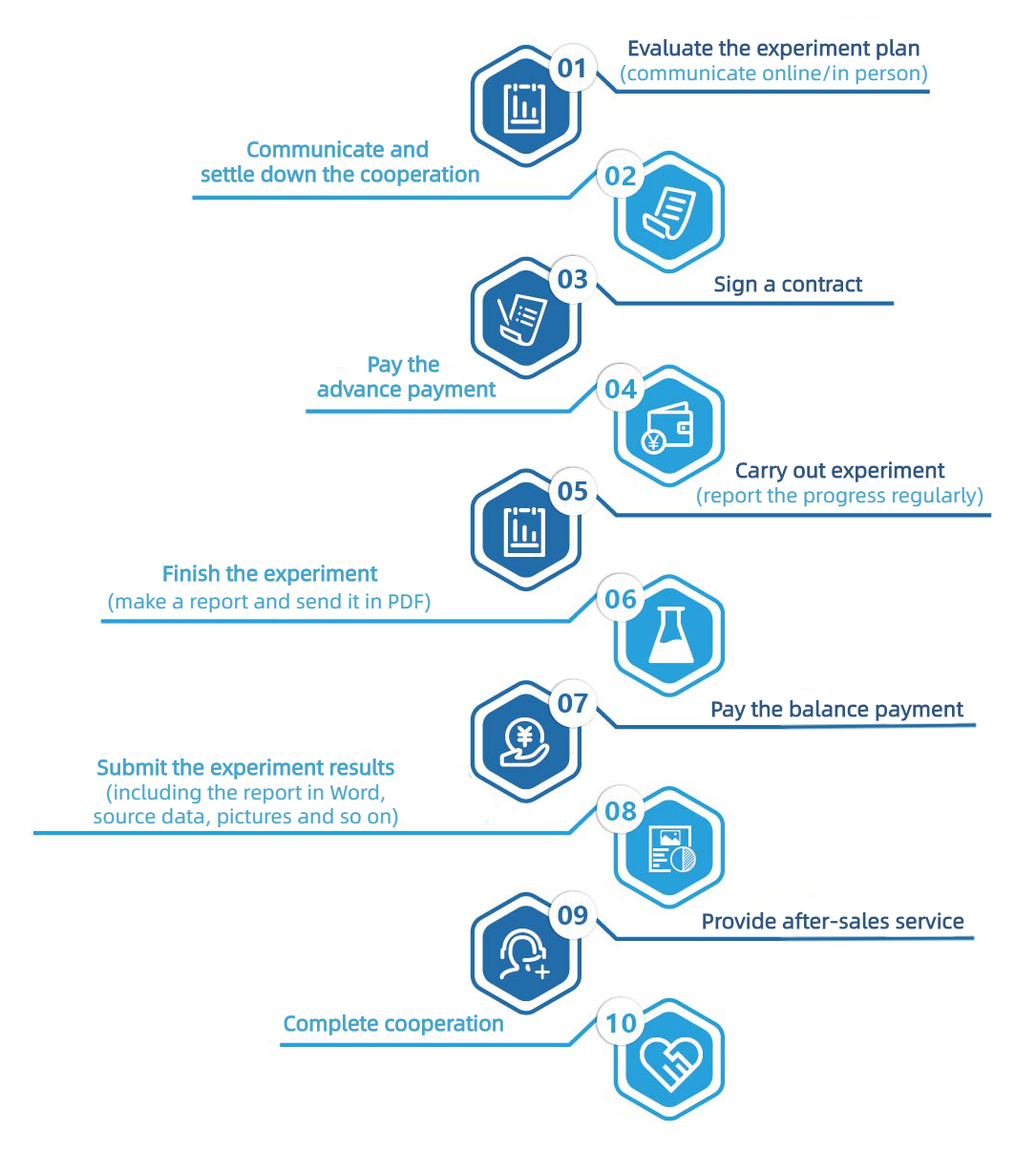
G. Service Process