One. Experiment Principle
The cell scratch method is a simple and direct method to determine the migration movement and repair ability of cells. Similar to the in vitro wound healing mode, it marks the central area of cell growth with amicro pipette tips or other hard objectson on the monolayer adherent cells cultured in vitro petri dish or plate, remove the cells in the central part, then continue to culture the cells until the time set in the experiment, take out the cell culture plate, and observe whether the surrounding cells grow to the central scratch area, so that to judge the growth and migration ability of cells. The experiment usually needs to set the normal control group and the experimental group. The experimental group is a group added with some treatment factors or drugs, exogenous genes, etc. through the repair ability of cells in different groups to the scratch area, the migration and repair ability of cells in each group can be judged.
Two. Application Introduction
Cell migration: also known as cell movement, cell crawling or cell movement, it is the movement of cells after receiving migration signals or feeling the gradient of some substances.
Cell migration is involved in many physiological and pathological activities, as follows:
1. Immunity: for example, the directional migration of lymphocytes is one of the keys to their differentiation, maturation and function;
2. Inflammation: the most important function of inflammation reaction is to send leukocytes to the inflammatory focus. Leukocytes migrate to the inflammatory site and play their role of phagocytosis and tissue damage;
3. Tumor metastasis: migration is one of the essential links in the process of tumor cell metastasis. When tumor cells separate from the parent tumor, cross the blood vessel wall and invade the surrounding normal tissues, they need a certain movement ability. Tumor cells with high metastasis usually have strong motility. A variety of substances can stimulate the migration of tumor cells, such as tumor cell secretory factors, growth factors, extracellular matrix components (FN, LN), the metabolites or secretory products of some cancer metastasis target organs. These growth factors have chemotaxis on tumor cells and can make them move directionally. Cell scratch method and transwell chamber method are used;
4. Damage repair: when the body is damaged, immune cells and platelets will migrate to the damaged site and participate in anti-inflammatory and coagulation;
5. Embryonic development: in the embryonic stage, different types of progenitor cells migrate to specific targets to ensure the normal development of various tissues and organs.
Three. Experiment Methods
Culture Insert method
1. Prepare cells, culture medium and culture insert.
2. As shown in the figure, inoculate the cells into the Insert in the middle of dish. After the cells grow all over the Insert area, remove the Insert with tweezers to produce 500 μ M wide scratches.
3. Take photos every 4-6 hours.
4. Analyze the experimental results according to the collected picture data.
Traditional experimental methods
1. Culture plate streaking
First, use a marker pen to draw a horizontal line evenly behind the 6-hole plate about every 0.5 ~ 1cm apart across the hole with a ruler. Each hole shall pass through at least 5 lines. Pay attention not to be too thick when streaking.
2. Laying cells
Add about 5 × 105 cells to the hole (the number of different cells is different, which is adjusted according to the growth speed of cells). The principle of inoculation is that the fusion rate reaches 100% after overnight.
3. Cell streaking
On the next day, scratch the cell layer with pipette tips head or sterile toothpick perpendicular to the cell plane along the line drawn on the back of the plate on the first day (it is best to use the same pipette tip or toothpick between different holes).
4. Wash cells
After the scratch is completed, wash the cells with sterile PBS for 3 times, wash away the cells that do not adhere to the wall, that is, the cells that are streaked when streaking, so that the gap left afterstreaking is clearly visible, and then replace the fresh serum-free medium.
5. Cell culture and observation
Put the cells into 37 ℃ 5% CO2 incubator and culture. Then take out the cells at an appropriate time point, such as 0, 6, 12 and 24h, observe and measure the width of the scratch with the microscope, and take photos (the specific time depends on the needs of the experiment).
Four. Samples Delivery Requirements
Sample type | Sample requirements | Preservation conditions | Delivery conditions | Note |
Frozen cell | 1. Take the cells in logarithmic growth stage, digest and centrifuge to collect the cells, add the cryopreservation solution, blow and mix well, put it into the cryopreservation tube, mark the cell name/cell algebra, and the number of frozen cells is 1-5*106 / ml 2. After the start of the project, the cells will be resuscitated. Report the cell status 3 days after resuscitation, report the cell contamination 3 to 5 days later and report the mycoplasma contamination 5 tp 7 days later. 3. Cell details (name, culture medium and other culture conditions, etc. if it has been specially treated, it is necessary to inform and provide necessary information) | In liquid nitrogen | With dry ice | All samples need to be uniquely marked and the markings are clearly identifiable |
Resuscitated cell | 1. Use T25 culture bottle for transportation. When the confluence of cells reaches more than 60%, fill the bottle with culture medium, leave only bubbles of button size, seal the bottle with sealing film, mark the cell name/cell Algebra/ inoculation time/medium type, and transport in bottle stably. 2. Report the cell status 3 days after receiving, report the cell contamination 3 to 5 days later and report the mycoplasma contamination 5 tp 7 days later. 3. Cell details (name, culture medium and other culture conditions, etc. if it has been specially treated, it is necessary to inform and provide necessary information) | At ambient temperature | At ambient temperature | |
Plasmid | 1. High purity, no endotoxin, no protein, genomic DNA / RNA pollution, the ratio of plasmid A260:A280 is between 1.8-2.0 2. The concentration is not less than 0.5 μg/μl, the total amount is more than 100 μg, and there is no endotoxin treatment 3. Carrier details, including name, band, resistance, fluorescent labeling | At - 20℃ | With ice bag | |
Virus | 1. Lentivirus: the titer is not less than 1*108 TU/ml, the volume is about 200 μl, the preparation time is indicated, and there is no repeated freezing and thawing 2. Adenovirus: the titer is not less than 1*109 PFU/ml, the volume is about 200 μl, the preparation time is indicated, and there is no repeated freezing and thawing 3. To determine whether to express fluorescent label protein and its type, it is best to provide virus vector map | At - 80℃ | With dry ice |
Five.Case Display
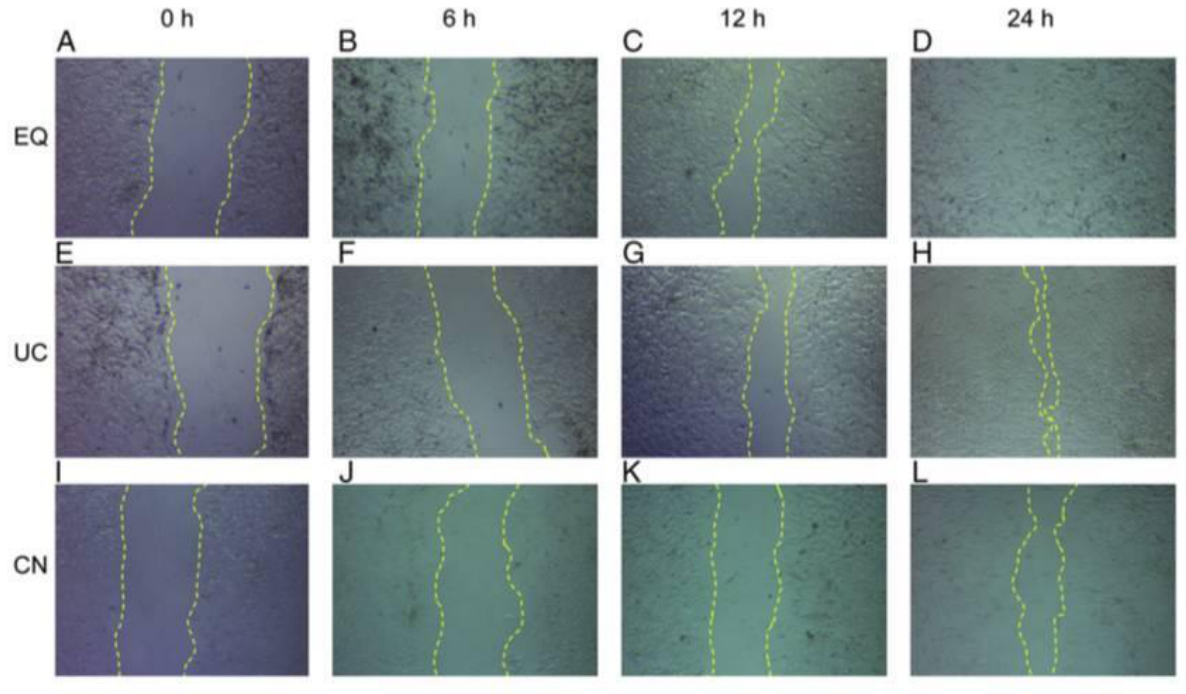
Six. Common Problems
1. It is best to use 6-well plate for cell laying, because 6 empty plates can ensure that there are straight scratches at a considerable distance, and because there are 5 positioning lines that intersect with the scratches, there are 10 fixable monitoring points without repetition and the error is very small. Of course, if high-throughput screening is required, 12 or 24 orifice plates can also be used.
2. If you monitor continuously for 24 hours, you need to know that the reduction of scratch is the result of the joint action of cell migration and cell reproduction, rather than simple cell migration. If you want to test cell migration alone, you can use Mitomycin (1) first μ G / ml) for one hour to inhibit cell division, so your result is the effect of cell migration. In addition, the use of serum-free medium can also reduce the effect of proliferation on the experimental results.
3. After the photos are taken, image J can be used to measure the pixels in the scratch area and quantitatively compare the speed of cell migration.
4. Although in serum-free culture, the effect of cell proliferation can be neglected, the speed of cell migration will be much slower due to the overall down regulation of intracellular signal transduction system.
5. When washing with PBS buffer, pay attention to adding slowly to avoid dispersing monolayer adherent cells.
6. In order to prevent the number and state of cells from being affected, try not to use a suction pump, but use a pipette tip to slowly remove them away.
Seven. Service Process