One. Experiment Principle
Stable transgenic cell line refers to a cell line constructed based on a cell line that continuously overexpresses or interferes with a specific gene. Lentivirus infection-drug screening method is a widely used method for the construction of stable transgenic strains, which has the characteristics of efficient integration and wide range of target cells.
The foreign DNA is cloned into a vector with certain resistance. The vector is transfected into the host cell and integrated into the host chromosome. The resistance markers contained in the vector are used for screening. The most commonly used resistance screening markers of eukaryotic expression vectors are neomycin, hygromycin and puromycin. Cell lines that can stably express the target protein or silence specific genes can be screened.
Two. Application Introduction
Stable transgenic plants are mainly used in the following studies:
1. Study that needs to study gene function in target cells for a long time. By constructing stable strains, the cost of frequent transfection or virus packaging can be greatly reduced, and it is also convenient for long-term experimental research;
2. The half-life of some proteins is extremely long, and transient RNA can only interfere with the expression, but can not remove the expressed target protein. The construction of stable strains can help to screen cells with an appropriate number of copies for experimental research;
3. Transient transfection may introduce the unexpected copy number expression (often high transient expression), leading to inaccurate experimental results due to human factors. The construction of stable strains can help to screen cells with appropriate copy number for experimental research;
4. Stable transgenic plants can be used to induce the experiment of expression system and control the spatiotemporal expression of genes;
5. Experiments that need to build animal models with target cells often need to build stable strains.
Three. Experiment Method
1. Screen the appropriate concentration of antibiotics of target cells;
2. Screen the appropriate virus titer of target cells;
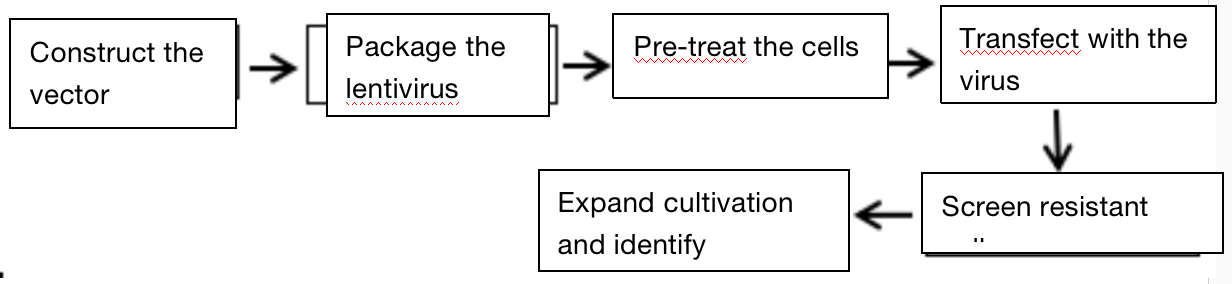
3. Construct the lentivirus, package and determine the titer;
4. Lentivirus infection;
5. Screen stable cell lines with specific antibiotics;
6. Identify the stable cell lines.
Four. Samples Delivery Requirements
Sample type | Sample requirements | Preservation conditions | Delivery conditions | Note |
Frozen cell | 1. Take the cells in logarithmic growth stage, digest and centrifuge to collect the cells, add the cryopreservation solution, blow and mix well, put it into the cryopreservation tube, mark the cell name/cell algebra, and the number of frozen cells is 1-5*106 / ml 2. After the start of the project, the cells will be resuscitated. Report the cell status 3 days after resuscitation, report the cell contamination 3 to 5 days later and report the mycoplasma contamination 5 tp 7 days later. 3. Cell details (name, culture medium and other culture conditions, etc. if it has been specially treated, it is necessary to inform and provide necessary information) | In liquid nitrogen | With dry ice | All samples need to be uniquely marked and the markings are clearly identifiable |
Resuscitated cell | 1. Use T25 culture bottle for transportation. When the confluence of cells reaches more than 60%, fill the bottle with culture medium, leave only bubbles of button size, seal the bottle with sealing film, mark the cell name/cell Algebra/ inoculation time/medium type, and transport in bottle stably. 2. Report the cell status 3 days after receiving, report the cell contamination 3 to 5 days later and report the mycoplasma contamination 5 tp 7 days later. 3. Cell details (name, culture medium and other culture conditions, etc. if it has been specially treated, it is necessary to inform and provide necessary information) | At ambient temperature | At ambient temperature | |
Plasmid | 1. High purity, no endotoxin, no protein, genomic DNA / RNA pollution, the ratio of plasmid A260:A280 is between 1.8-2.0 2. The concentration is not less than 0.5 μg/μl, the total amount is more than 100 μg, and there is no endotoxin treatment 3. Carrier details, including name, band, resistance, fluorescent labeling | At - 20℃ | With ice bag | |
Virus | 1. Lentivirus: the titer is not less than 1*108 TU/ml, the volume is about 200 μl, the preparation time is indicated, and there is no repeated freezing and thawing 2. Adenovirus: the titer is not less than 1*109 PFU/ml, the volume is about 200 μl, the preparation time is indicated, and there is no repeated freezing and thawing 3. To determine whether to express fluorescent label protein and its type, it is best to provide virus vector map | At - 80℃ | With dry ice |
Five. Case Display
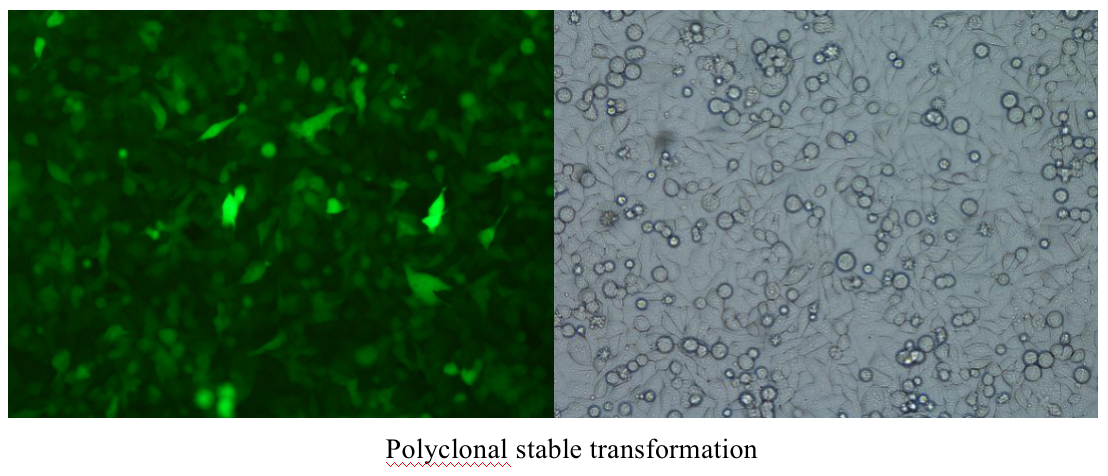
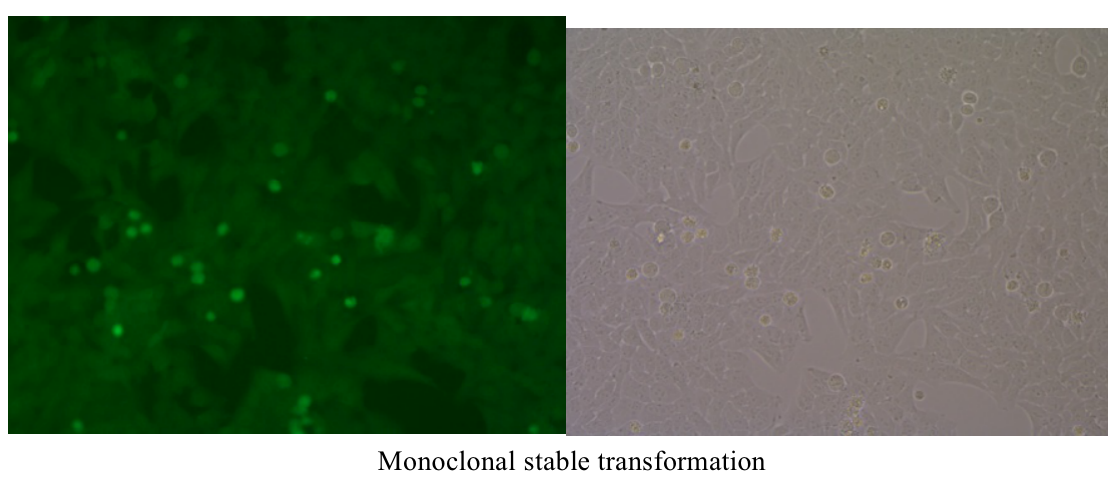
Six. Common Problems
1. Mycoplasma contamination
Because mild mycoplasma contamination does not affect cell growth and proliferation, it is ignored by many laboratories. However, mycoplasma is easy to break out after virus infects cells, resulting in a large number of cell fragments and even cell death, resulting in the failure of stable transformation screening. We suggest that mycoplasma contamination in cells and culture environment should be eliminated at the beginning of the construction of stable transgenic strains.
2. Other issues
In addition to mycoplasma contamination, the following problems often occur in the construction of stable transgenic plants.
Problem | Cause | Solution |
No target gene is detected | Initiation factor silencing | 1. Integration into chromosome may silence CMV promoter. It can screen multi-drug resistant clones and select the clones with the strongest expression. 2. pLenti/UbC/V5-DEST constrcut lentivirus vector |
The storage of lentivirus is inappropritate | Pack separately and store at -80°C,it can not be repeatedly frozen and thawed for 3 times | |
Low expression level of target gene. | Low infected efficiency: 1. No infecting enhancer is used 2. Host cells are non-dividing cells | 1. Use infecting enhancer. 2. Increase the MOI |
Low MOI | Increase MOI | |
Over-use the drug for resistance screening. | It needs to prepare the antibiotic death curve and select the appropriate antibiotic concentration. | |
Collect the cell prematurely after infection. | Test 48-72 h after infectio | |
The target gene is toxic to cells | Overexpression of potentially harmful genes or knockdown of important genes are not recommended | |
High toxicity after infection leads to cell death | The amount of virus is excessive during infection. | 1. Change the solution immediately 2. Purify the virus |
The amount of infecting enhancer is excessive | Perform gradient experiment to determine safe dose of infecting enhancer | |
Resistance screening drug overdose. | It needs to prepare the antibiotic death curve and select the appropriate antibiotic concentration. | |
The target gene is toxic to cells. | Try to use other cell lines |
Seven. Service Process