Recently, researchers from the School of Pharmacy of East China University of Science and Technology have made new progress in the research on the regulation mechanism of autophagy, and related research has been published in Science Advances.

Autophagy is an important process for Eukaryote to turnover intracellular substances. Autophagy plays an important role in physiological and pathological processes, including adaptation to metabolic stress, elimination of protein aggregates and damaged Organelle, preimplantation development of embryos, aging, Degenerative disease, occurrence and development of tumors, etc. The regulation of autophagy and its role in physiology and pathology has always been a research hotspot in this field.
In this study, researchers found through experiments that a kinase (ULK1) enhances its activity and promotes lactate secretion by phosphorylating Lactate dehydrogenase A (LDHA). Lactic acid is catalyzed by acyltransferase to mediate the lactate modification of type III Phosphatidylinositol kinase (Vps34) lysine. The lactated modification of Vps34 significantly promotes the interaction of its complex subunits, improves its kinase activity, and further promotes autophagy and endosome Lysosome degradation pathway.
In addition, this study elucidated the effects of Vps34 lactate on muscle homeostasis and tumor progression through mouse muscle movement models and clinical tumor samples. Under physiological conditions, muscle lactate occurs during intense exercise, increasing the level of autophagy in muscle tissue cells and maintaining the steady state of muscle tissue during exercise. Under pathological conditions, the high level of lactic acid produced by the aerobic Glycolysis of tumor tissue acts as a Signaling molecule, which can improve the autophagy of tumor cells and promote tumor progress by regulating its lactate.
To sum up, this research achievement clarifies the molecular mechanism of ULK1 phosphorylation regulating LDHA activity, clarifies the effect of lactic acid mediated Vps34 on autophagy and endosome Lysosome degradation pathway, reveals the effect of Vps34 lactate on muscle homeostasis and tumor progression, integrates the Glycolysis regulation pathway and cell autophagy regulation pathway, and shows the diversity and complexity of autophagy regulation in higher mammals.
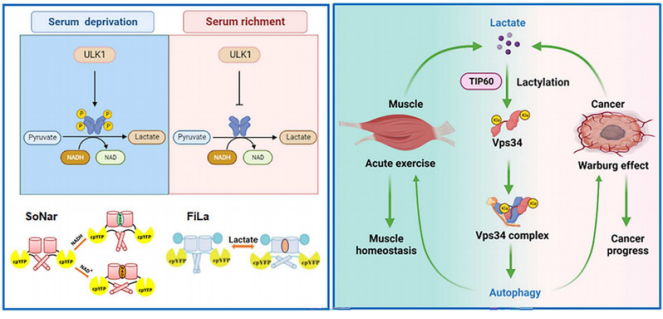
ULK1-mediated metabolic reprogramming regulates Vps34 lipid kinase activity by its lactylation
Journal: Science Advances
Corresponding Authors: Yi Yang, Yuzheng Zhao, Xiawei Cheng
Abstract:
Autophagy and glycolysis are highly conserved biological processes involved in both physiological and pathological cellular programs, but the interplay between these processes is poorly understood. Here, we show that the glycolytic enzyme lactate dehydrogenase A (LDHA) is activated upon UNC-51–like kinase 1 (ULK1) activation under nutrient deprivation. Specifically, ULK1 directly interacts with LDHA, phosphorylates serine-196 when nutrients are scarce and promotes lactate production. Lactate connects autophagy and glycolysis through Vps34 lactylation (at lysine-356 and lysine-781), which is mediated by the acyltransferase KAT5/TIP60. Vps34 lactylation enhances the association of Vps34 with Beclin1, Atg14L, and UVRAG, and then increases Vps34 lipid kinase activity. Vps34 lactylation promotes autophagic flux and endolysosomal trafficking. Vps34 lactylation in skeletal muscle during intense exercise maintains muscle cell homeostasis and correlates with cancer progress by inducing cell autophagy. Together, our findings describe autophagy regulation mechanism and then integrate cell autophagy and glycolysis.





