Scientists from China have recently analyzed the three-dimensional structure of key protein synthesis machines in fungal cell walls and revealed novel potential mechanisms of action of antifungal drugs.

β- 1,3-glucan is a unique core component of fungal cell walls and plays a crucial role in the viability of various pathogenic fungi. Echinococcins, which was developed to destruct the β- 1,3-glucan, have been widely used as first-line anti-fungal drugs in clinical practice since their advent in the 19th century. However, a series of problem, like the synthesis mechanism of the β- 1,3-glucan of the fungal cell wall, the mechanism of action of Echinocandins as anti-fungal drugs, and the widespread sources of drug resistance in clinical practice, have seriously obstructing the target β- 1,3-glucandevelopment of novel antifungal drugs synthesized from 1,3-glucan.
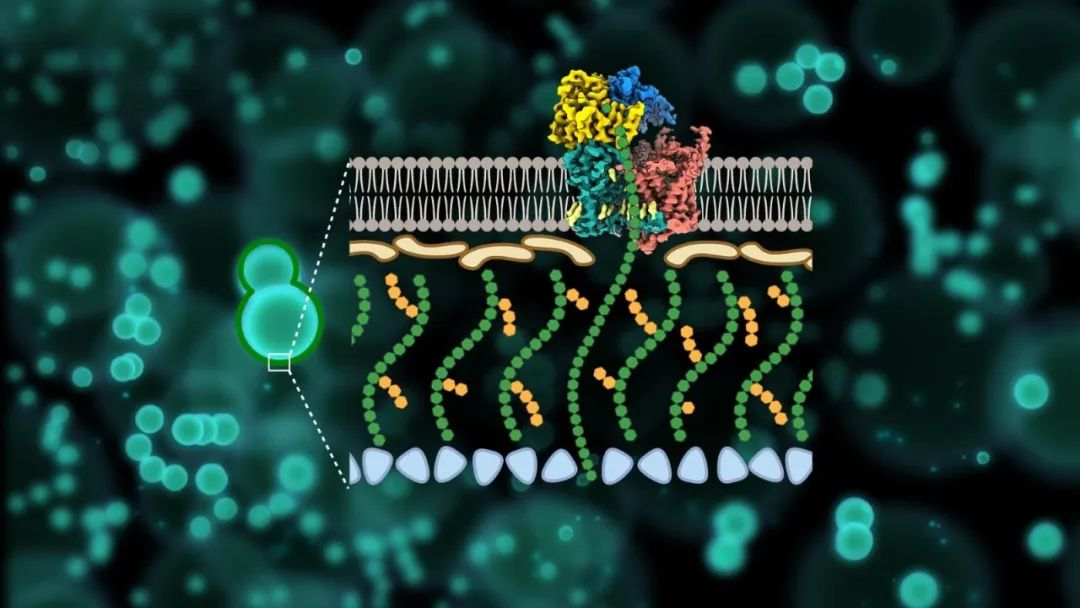
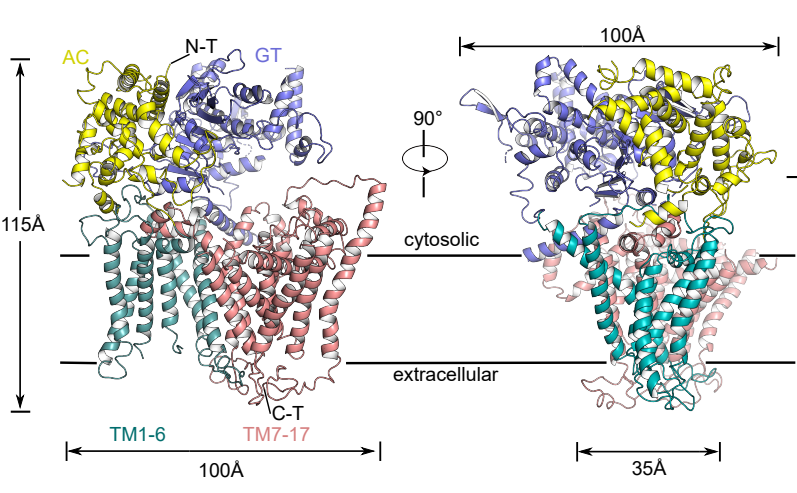
To address the above questions, the research team used single-particle cryo-electron microscopy (Cryo-EM) to resolve the high-resolution structure (3.4 Å) of the fungal β-1,3-glucan synthase FKS1, reporting for the first time its unique molecular panoply and revealing its key components involved in β-1,3-glucan synthesis. Systematic mutagenesis combined with in vivo and in vitro functional analysis further validated the catalytic synthesis and transport mechanism of FKS1 and revealed key amino acid sites conserved in a variety of pathogenic fungi.
Next, the researchers resolved the high-resolution electron microscopic structure (3.5 Å) of the most common representative clinical drug resistance mutant FKS1-S643P. Structure comparison analysis revealed that mutation at this site causes significant conformational changes in Y638 and F639 in the hotspot region of the drug resistance mutation, and ultimately causes rearrangement of nearby lipid molecules, suggesting a unique molecular mechanism for the generation of drug resistance.
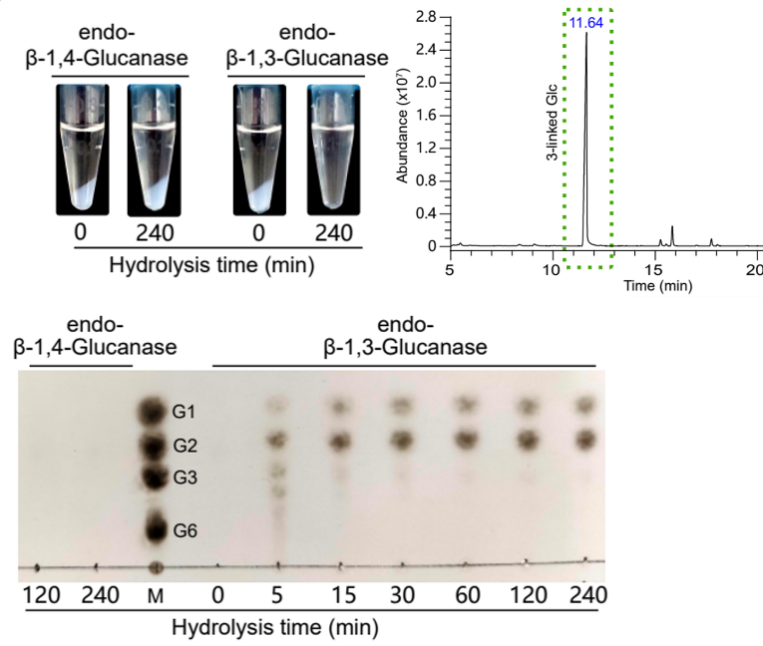
More importantly, the research team has successfully identified the important mutations affecting FKS1 the activity, as well as the key purification and reaction conditions. They also successfully established an efficient and reproducible in vitro reaction system for FKS1 family proteins, eliminating the cumbersome radiolabeling assay for the first time. This greatly optimizes the drug screening system and fills a gap in the field.
https://www.nature.com/articles/s41586-023-05856-5
Structural and mechanistic insights into fungal β-1,3-glucan synthase FKS1
Journal: Nature
Abstract:The membrane-integrated synthase FKS is involved in the biosynthesis of β-1,3-glucan, the core component of the fungal cell wall1,2. FKS is the target of widely prescribed antifungal drugs, including echinocandin and ibrexafungerp3,4. Unfortunately, the mechanism of action of FKS remains enigmatic and this has hampered development of more effective medicines targeting the enzyme. Here we present the cryo-electron microscopy structures of Saccharomyces cerevisiae FKS1 and the echinocandin-resistant mutant FKS1(S643P). These structures reveal the active site of the enzyme at the membrane–cytoplasm interface and a glucan translocation path spanning the membrane bilayer. Multiple bound lipids and notable membrane distortions are observed in the FKS1 structures, suggesting active FKS1–membrane interactions. Echinocandin-resistant mutations are clustered at a region near TM5–6 and TM8 of FKS1. The structure of FKS1(S643P) reveals altered lipid arrangements in this region, suggesting a drug-resistant mechanism of the mutant enzyme. The structures, the catalytic mechanism and the molecular insights into drug-resistant mutations of FKS1 revealed in this study advance the mechanistic understanding of fungal β-1,3-glucan biosynthesis and establish a foundation for developing new antifungal drugs by targeting FKS.





