Scientists from China revealed a new mechanism of immune evasion of liver metastases and proposed targeted therapies
Recently, A team from the University of Science and Technology of China published in the Journal of Clinical Investigation with the title “In situ expansion and reprogramming of Kupffer cells elicits potent tumoricidal immunity against liver metastasis”.

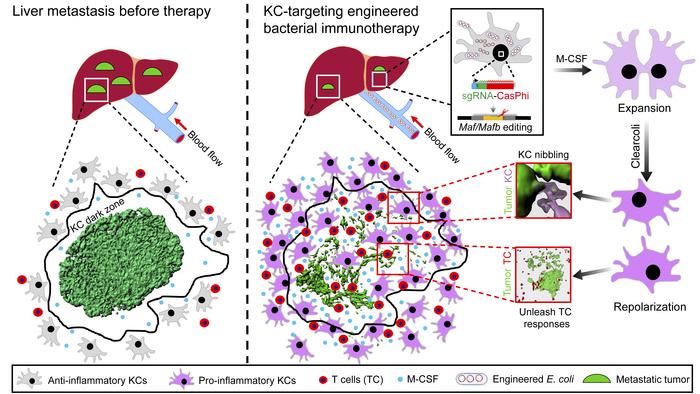
Led by Zhutian Zeng, the research group revealed the new mechanism of liver metastasis escaping from immune surveillance of Kupffer cells (KC). In addition, a new method of in situ targeted amplification and remodeling of KC anti-tumor function has been developed, and tumor clearance has been achieved in a variety of animal models of end-stage liver metastasis. This provides a new idea for the development of immunotherapy strategies for metastatic liver cancer.
Schematic principle of Kupffer cell in situ gene editing for treatment of liver metastasis
In situ expansion and reprogramming of Kupffer cells elicits potent tumoricidal immunity against liver metastasis
First Authors: Wei Liu, Xia Zhou
Journal: Journal of Clinical Investigation
Abstract
Liver metastasis represents one of the most frequent malignant diseases with no effective treatment. As the largest population of hepatic macrophages, functional reprogramming of Kupffer cells (KCs) holds promise for treating liver cancer but remains seldom exploited. Taking advantage of the superior capacity of KCs to capture circulating bacteria, we report that a single administration of attenuated Escherichia coli producing CRISPR‒CasΦ machinery enables efficient editing of genes of interest in KCs. Using intravital microscopy, we observed a failure of tumor control by KCs at the late stage of liver metastasis due to KC loss preferentially in the tumor core and periphery, resulting in inaccessibility of these highly phagocytic macrophages to cancer cells. Simultaneous disruption of MafB and c-Maf expression using the aforementioned engineered bacteria could overcome KC dysfunction and elicit remarkable curative effects against several types of metastatic liver cancer in mice. Mechanistically, bacterial treatment induced massive proliferation and functional reprogramming of KCs. These cells infiltrated into the tumor, dismantled macrometastases by nibbling cancer cells, and skewed toward proinflammatory macrophages to unleash antitumor T-cell responses. These findings provide an immunotherapy strategy that could be applicable for treating liver metastasis and highlight the therapeutic potential of targeting tissue-resident macrophages in cancer.
https://doi.org/10.1172/JCI157937





